Abstract
The goal of this laboratory exercise is to increase student understanding of the impact of nervous system function at both the organismal and cellular levels. This inquiry-based exercise is designed for an undergraduate course examining principles of cell biology. After observing the movement of Caenorhabditis elegans with defects in their nervous system, students examine the structure of the nervous system to categorize the type of defect. They distinguish between defects in synaptic vesicle transport and defects in synaptic vesicle fusion with membranes. The synaptic vesicles are tagged with green fluorescent protein (GFP), simplifying cellular analysis. The expected outcome of this experiment is that students will better understand the concepts of vesicle transport, neurotransmitter release, GFP, and the relation between the nervous system and behavior.
Keywords: vesicle transport, vesicle fusion, synaptic vesicles, green fluorescent protein, fluorescence microscopy, Caenorhabditis elegans , undergraduate laboratory exercise
INTRODUCTION
We pursued an inquiry-based exploration of the relationship between nervous system function and animal movement in an introductory undergraduate cell biology course. This article describes an exercise designed to enhance education in biology by encouraging student exploration and critical thinking, while introducing important biological concepts. Such an approach has been recommended by the Division of Undergraduate Science, Engineering, and Mathematics Education (1989). Specifically, students observe the movement of worms with nervous system defects and then examine the structure of the nervous system to categorize the type of defect. They distinguish between defects in vesicle transport and defects in vesicle fusion with membranes. Students can utilize two different types of microscopy in this study; they can examine movement of the worms with a stereomicroscope, followed by analysis of their nervous systems at the cellular level using a fluorescent microscope. Two aspects of the experiment make it easily accessible to undergraduate students in an introductory cell biology course. First, the exercise utilizes the nematode, Caenorhabditis elegans, an organism that is easy to observe, maintain, and manipulate. Second, the nervous system of the worm is tagged with green fluorescent protein (GFP), simplifying cellular analysis. By applying concepts learned in a cell biology course, students are expected to attain a deeper understanding of cell biology (National Research Council, 1997). As a result of this exercise, students are expected to achieve a greater understanding of vesicle transport, neurotransmitter release, GFP, and the relation between the nervous system and behavior.
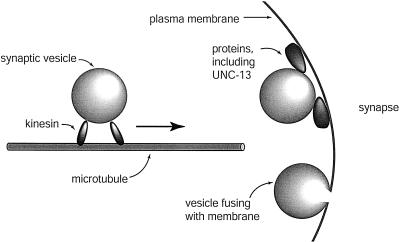
Neurons send signals to other neurons or to muscle cells by releasing neurotransmitters. Neurotransmitters are chemicals that diffuse through the space between the cells and stimulate a response in the adjacent cell. In this manner, a neuron can stimulate a muscle to contract. Before they are released, the neurotransmitters are packaged into vesicles in the cell bodies of the neurons and transported down the length of the axon. The vesicles accumulate at the end of a neuron until a signal stimulates the vesicles to fuse with the cell membrane and release their contents into the synapse (Figure 1).
Figure 1.
Vesicle transport and fusion. The motor protein kinesin is shown moving a vesicle along a microtubule. Vesicles are depicted docked at the plasma membrane and fusing with the plasma membrane at a synapse.
Defects in neurotransmitter release result in improper stimulation of muscle cells, altering movement. Two events in neuronal signaling that can interrupt nervous system signals are defects in vesicle transport along the axon and defects in vesicle fusion with the cell membrane. Students examine worms with each of these types of defects as well as worms with a normal nervous system. The worms with defects in vesicle transport have a mutation in the gene coding for a motor protein that drags the vesicles down the length of the axon. This motor protein, kinesin, is found in many organisms, including mammals (Hall and Hedgecock, 1991; Otsuka et al., 1991). The worms with defects in vesicle fusion have a mutation in gene coding for a protein that regulates fusion of synaptic vesicles with the cell membrane. The protein is called UNC-13 and is homologous to proteins in mammals that are also important in nervous system function (Brose et al., 1995; Richmond et al., 1999).
Students are first asked to design an experiment to determine which worms have a defect in nervous system function and which worms have a normal nervous system. If students make the connection that a defect in the nervous system would affect movement of the worm, then they can design a simple experiment observing movement of the different strains of worms using a dissecting microscope. Worms with a normally functioning nervous system would move quickly on a plate, while worms with a defect in nervous system function would move slowly or not at all.
After distinguishing between normal and mutant worms based on movement of the organisms, students are asked to design an experiment to determine what type of nervous system defect is affecting the worm's behavior. The nervous system in the worms has been labeled with the fluorescent tag GFP, which causes bioluminescence in the Pacific Northwest jellyfish, Aequorea victoria. After GFP absorbs blue light, it emits green light. GFP can be incorporated into other living organisms and used as a fluorescent tag (Chalfie et al., 1994). In this study, GFP is attached to a protein on synaptic vesicle membranes (Nonet, 1999). Using this knowledge, students can design an experiment in which they compare the appearance of the nervous system in normal worms to the appearance of the nervous system in mutant worms. Because the location of synaptic vesicles in neurons can be identified by a green glow visualized with a fluorescent microscope (Figure 2), students may choose to observe the worms under the fluorescent microscope and distinguish between vesicle transport and vesicle fusion mutants based on the localization of GFP in the nervous system. As they have already determined which worms have a normal nervous system, they have established a control for their study of the subcellular localization of synaptic vesicles in the mutant worms.
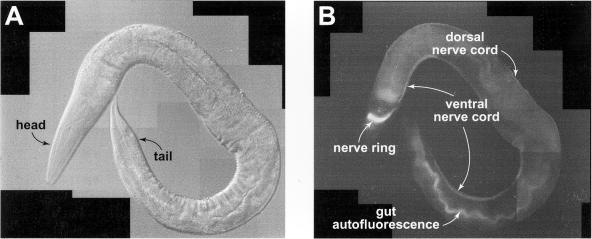
Figure 2.
Caenorhabditis elegans nervous system. (A) An adult C. elegans worm (NM1233) is shown using differential interference contrast microscopy. (B) The same worm is shown using fluorescence microscopy. The nervous system of the worm glows due to the presence of GFP. The nerve ring, ventral nerve cord, and dorsal nerve cord are visible.
Caenorhabditis elegans is an ideal organism for studying the nervous system in an undergraduate laboratory. The worms are simple, nonparasitic, and develop quickly. Because they are only 1 mm long, the plates they grow on take up little space. Populations can be frozen long term, so that a stock can be thawed shortly before the laboratory exercise. Moreover, the nervous system of C. elegans has been studied and characterized extensively at the cellular level (White et al., 1986; Hall and Russell, 1991). Surprisingly, the nervous system of this small worm functions in many ways like our own nervous system (Bargmann, 1998). Genes that are involved in human neurological disorders, such as Alzheimer's disease, have counterparts in C. elegans (Levitan and Greenwald, 1995; McDermott et al., 1996). Using C. elegans in a laboratory exercise presents an opportunity to emphasize the importance of model organisms in basic research.
The following methods were designed to make this experiment feasible for a large introductory cell biology course for undergraduates. During a semester, we found it possible to run six laboratory sections, each with approximately 20 students. The students worked in groups of four to enhance cooperative learning in the laboratory (Johnson et al., 1991; Advisory Committee to the National Science Foundation Directorate for Education and Human Resources, 1996; Strum-Kenny, 1998). The necessary equipment for this laboratory exercise should be found in most colleges or universities.
MATERIALS AND METHODS
Preparations to Make Several Weeks in Advance
The appropriate C. elegans strains and the bacteria for feeding C. elegans can be obtained from the Caenorhabditis Genetics Center (CGC; Rochester, MN). The C. elegans strains NM1233, NM440, and RK001 have GFP-labeled synaptic vesicles. NM1233 has a normal nervous system, NM440 has a defect in vesicle transport, and RK001 has a defect in vesicle fusion. The bacterial strain, OP-50, is a strain of Escherichia coli used as food for C. elegans. Send your request by e-mail to stier@biosci.cbs.umn.edu. Indicate the names of the strains you would like to receive, a one-sentence description of what you would do with the strain, and your complete mailing address and phone number. The strains are sent through the mail on agar plates and should arrive in about 7–10 days, plus shipping time. The CGC is supported by funds from the National Institutes of Health (NIH); therefore, educational or nonprofit organizations are not charged for the strains or for shipping.
- 2× TY medium for growing bacteria is prepared (another rich medium can be substituted; Ausubel et al., 1998).
- Liquid medium for growing bacterial cultures:
Tryptone 16 g Yeast extract 10 g NaCl 5 g -
Solid medium for bacteria plates:The same dry ingredients used to make the liquid medium are mixed together and then 15 g of agar are added. The volume is brought to 1 L with dH2O. The solution is autoclaved. After the medium cools to approximately 60°C, it is poured into 100 mm petri dishes.
OP-50 bacteria for feeding C. elegans is grown. Streak OP-50 on a plate with 2× TY medium. The bacteria is grown overnight at 37°C or at room temperature for 48 h. A single colony is transferred to a screw cap bottle with liquid 2× TY using sterile technique. The cells are allowed to grow at room temperature overnight. The culture can be stored at 4°C for a month.
OP-50 bacteria are frozen for long-term storage. Transfer 700 μl of bacterial culture to a sterile plastic tube. Add 300 μl of sterile 50% glycerol. Freeze at −80°C. To revive the strain, scrape a sterile applicator across the frozen bacteria and streak on a 2× TY agar plate.
- NGM plates for growing C. elegans are prepared (Sulston and Hodgkin, 1988).
NaCl 3 g Agar 17 g Peptone 2.5 g Cholesterol (5 mg/ml in EtOH) 1 ml H2O 975 ml 1 M CaCl2 1 ml 1 M MgSO4 1 ml 1 M Potassium phosphate pH 6 25 ml NGM plates are streaked with OP-50 bacteria. A glass stir rod is dipped in EtOH and then passed through the flame of a Bunsen burner. Using sterile technique, the stir rod is dipped into the OP-50 bacterial culture stored at 4°C. The stir rod is then dragged across the surface of two NGM agar plates. The process is repeated until all the plates are streaked. Allow the bacteria to grow overnight at room temperature with the lids on the plates. The plates are stored upside down in plastic bags at 4°C. When streaking plates, be sure not to break the surface of the agar plate.
Worms are transferred to new plates. A small flat spatula is dipped in EtOH and then passed through the flame of a Bunsen burner. The spatula is allowed to cool for a few seconds without touching anything. The sterile spatula is used to cut out a chunk of agar from a plate with worms. The chunk should be approximately 1 cm by 1 cm. The chunk of agar is transferred to an NGM agar plate with a streak of bacteria. The worms are allowed to grow at room temperature for several days. Different strains of worms grow at different rates. Strain NM1233, which has a normal nervous system, will grow the fastest. The plate should be covered with worms in 3 or 4 days. Strains RK001 and NM440 have mutations affecting their nervous systems and could take one or two weeks for worms to cover the plates.
-
The worms can be stored long term for future laboratory exercises (Sulston and Hodgkin, 1988). Grow two NGM agar plates with a strain of worms until the day after the bacteria are consumed. M9 buffer and freezing solution is prepared.
- M9 buffer
KH2PO4 3 g Na2HPO4 6 g NaCl 5 g 1 M MgSO4 1 ml H2O 1 L - Freezing solution
NaCl 5.685 g KH2PO4 6.8 g Glycerol 300 g 1 M NaOH 5.6 ml H2O to 1 L
One milliliter of M9 buffer is added to each plate. The M9 with worms is transferred to a sterile plastic tube using a sterile Pasteur pipette. An equal volume of freezing solution is added to the tube. The amount of 0.5 ml aliquots are transferred to plastic tubes for freezing. The tubes are frozen at −80°C. To thaw the worms, warm one plastic tube until it is just thawed. Pour the contents of the tube onto an NGM agar plate with bacteria. After the worms are growing well, transfer some to a new plate.
Materials to Gather a Week Before the Laboratory Exercise
At least one plate of a C. elegans strain per lab group plus one plate of each strain labeled “Observation”
Stereomicroscope illuminated from the base: one for the class to share, or one per lab group
Distilled water
Pasteur pipettes and bulbs
Microcentrifuge (optional)
1.5 ml snap cap tubes if using a microcentrifuge, or any small tube
Microscope slides and cover slips
Glycerol
Fluorescent microscope
Techniques Students May Choose to Use During the Laboratory Exercise
Agar plates with worms can be placed directly under a dissecting stereomicroscope without any other preparation. When observing C. elegans, do not leave a plate of worms over the light for very long. If the worms heat up, they will become sick.
-
Worms can be transferred to microscope slides for fluorescence microscopy. Add enough water to just cover the plate of worms. Swirl the water gently and transfer the fluid with worms to a small tube using a Pasteur pipette. If a microcentrifuge is available, spin the tubes for 1 min. If a microcentrifuge is not available, allow the worms to settle to the bottom of the tube for about 5 min. Remove most of the water without removing the worms. Add approximately 1 ml of water to the tube to wash away bacteria. Spin the tube in a microcentrifuge, or allow the worms to settle. Remove most of the water with a Pasteur pipette and repeat the wash. In the end, there should be a pellet of worms in the bottom of the tube with a small amount of liquid. Add two drops of glycerol to the worm pellet. Use a Pasteur pipette to transfer a drop of worms to a microscope slide. Gently lower a coverslip over the worms.
When working with a large lab section, each lab group can be given one plate with one strain of worms. Three lab groups, each with a slide of a different strain of worm, can then observe the slides together with the fluorescent microscope.
Worms can be observed under the fluorescent microscope. First, the mercury lamp on the microscope is warmed up for 15 min before use. A sliding barrier prevents the light from reaching the specimen until the student is ready to observe the fluorescence. The worms are located on the slide using bright field microscopy. After the worm is in focus using the 40× objective, the bright field light source is covered. Next, the barrier that blocks the light from the mercury bulb from reaching the sample is slid away. The blue cube is slid into the path of the light so that the sample is excited by blue light. The pattern of fluorescence in the worm is observed.
RESULTS
The Effect of Nervous System Defects on Organismal Behavior
Students designed experiments to determine which worms had nervous system defects and which worms had normally functioning nervous systems. Most students quickly grasped the idea that a worm with a defect in its nervous system would be unable to signal its muscles to produce normal movement. Since students were working in groups of four, they often came to this conclusion through group discussion. Most groups, therefore, chose to analyze the behavior of the worms by comparing the movement of different strains. Using stereomicroscopes, observation of worm movement was straightforward. After a few minutes of observation, students could distinguish between the fast movement of the worms with a normal nervous system and the extremely slow movement of the worms with mutations affecting the nervous system. All the worm strains carried a mutation in the cuticle, which caused them to move in circles. Students observed that the C. elegans strain NM1233 moved fastest, and that strains RK001 and NM440 moved slowly. The groups then deduced that NM1233 must have a normally functioning nervous system, while RK001 and NM440 had some defect in nervous system function. The results of the first experiment, however, did not allow them to determine what types of defects inhibited nervous system function.
Determining Types of Nervous System Defects
Students next designed experiments to determine what types of defects were present in the nervous systems of C. elegans strains RK001 and NM440. The students were aware from discussions and readings in their cell biology course that synaptic vesicles must travel to the appropriate location in a neuron and fuse with the plasma membrane to release neurotransmitter. They also knew that the synaptic vesicles of all the worms they were studying were tagged with GFP and that they had access to a fluorescence microscope. The second experiment asked them to analyze a complex phenomenon: how nervous system function is affected by different defects in neurons. Because the concepts were complex, group discussions were critical for experimental design. By working in a group, students pooled their basic knowledge of nervous system function and their awareness of the tools available, and chose to design experiments to observe the pattern of GFP in the worms using the fluorescent microscope. Because they had already deduced that the nervous system of strain NM1233 was normal, they used the appearance of its nervous system as a control for normal distribution of synaptic vesicles.
Using the fluorescent microscope to observe cellular structure was somewhat challenging for the students. The microscope was larger and more complex than ones with which the students were familiar. Therefore, a longer time was needed to find worms for observation than with the stereomicroscope. One suggestion to make the microscope observation run efficiently would be to have three lab groups each bring a slide to the fluorescent microscope at one time. The laboratory instructor can arrange that slides of NM1233, NM440, and RK001 worms are each brought to the fluorescent microscope by a different group. If one person from each lab group finds a worm on their slide with bright field microscopy and then switches the microscope to fluorescence microscopy, each student in the room can then take a turn observing the green glow in the nervous system. In this manner, three worm strains can be observed using one fluorescent microscope and the lab groups can collect data for all the strains in a short period of time.
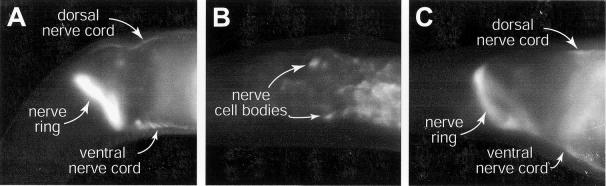
Students observed that the pattern of GFP in the nervous system, which represented the location of synaptic vesicles, varied for different strains. In the control for a normal nervous system, strain NM1233, they visualized the nerve ring, and ventral and dorsal nerve cords (Figure 3). Although the vesicles are found at synapses, the synapses are close enough together that the nerve ring and nerve cords appear as lines under a low magnification, rather than as punctate spots. When students observed C. elegans strain RK001, which they had already determined had a defect in nervous system function, they saw the same pattern of GFP as in NM1233 (Figure 3). Students discussed the idea that a worm with a defect in nervous system function could still have synaptic vesicles located at synapses. They deduced that the vesicles must be transported properly along axons but were unable to release neurotransmitter. Therefore, they determined that strain RK001 represents a mutation in the gene unc-13, which regulates vesicle fusion. When students observed the GFP pattern in strain NM440, they visualized a different pattern. In these worms, nerve cells bodies were visible in the head as small ovals. Some cell bodies were visible along the ventral nerve cord, but the thin line representing synapses along the ventral nerve cord was no longer visible. The dorsal nerve cord was no longer visible (Figure 3). In young larvae, the cell bodies in the ventral nerve cord may be close enough together to look like a line (data not shown). Students knew that the strain had a defect in nervous system function and that the vesicles must not be arriving at the appropriate location in neurons. Through group discussion, they concluded that the vesicles must not be transported properly, as would be consistent with a mutation in unc-104, which produces a motor protein needed for vesicle trafficking along axons. By combining data from two experiments that they designed, students determined which worms had defects in their nervous systems, and what type of defect at the subcellular level prevented the organism from moving properly.
Figure 3.
The pattern of GFP in the nervous system varies for different strains. (A) The head of an adult NM1233 worm is shown. The nerve ring, and ventral and dorsal nerve cords, are all visible. (B) The head of an adult NM440 worm is shown. Cells bodies are visible in the head as small ovals. The thin line representing synapses along the ventral nerve cord is no longer visible. (C) The head of an adult RK001 worm is shown. The nervous system appears the same as in strain NM1233.
Assessment of the Exercise
Student response to the exercise was evaluated for six laboratory sections. Each section was part of a first-year cell biology course. For quantitative analysis, a Likert-scale questionnaire (Table 1) was adapted from the “Student Assessment of Learning Gains” website (Seymour, 1997). The website is a free site that is designed to help instructors gain feedback about how elements of their courses help students to learn. For qualitative analysis, students were asked directed questions to assess how their knowledge of cell biological topics improved (Table 2 and Figure 4). Both types of assessment indicated an increase in understanding of cell biological topics.
Table 1.
Quantitative assessment of vesicle transport and fusion laboratory experimenta
| Mean ± SD | Mean ± SD | |
| Question | (first time taughtb) | (taught previouslyc) |
|
| ||
| Q1: How much did each of the following aspects of the laboratory exercise help your learning? | ||
| A. The way in which the material was approached | 2.5 ± 0.8 | 1.9 ± 0.6d |
| B. How the laboratory activity connected with ideas in the lecture | 2.4 ± 1.0 | 1.6 ± 0.7d |
| C. The laboratory activity | ||
| 1. Written lab instructions | 2.5 ± 1.1 | 1.9 ± 0.7e |
| 2. Lab organization | 2.6 ± 1.0 | 1.8 ± 0.6d |
| 3. Teamwork in the lab | 2.3 ± 1.1 | 1.7 ± 0.7e |
| D. The mental stretch required to understand the exercise | 2.8 ± 1.0 | 2.0 ± 0.8d |
| E. The overall way this laboratory exercise was presented | 2.6 ± 1.0 | 1.7 ± 0.7d |
| Q2: As a result of your work in this exercise, how well do you think that you now understand each of the following? | ||
| A. Vesicle transport | 2.5 ± 1.1 | 1.7 ± 0.7d |
| B. Neurotransmitter release | 2.4 ± 1.0 | 1.7 ± 0.7e |
| C. The affect of the nervous system on behavior | 2.6 ± 1.1 | 2.2 ± 0.8e |
| D. GFP | 2.7 ± 1.2 | 2.1 ± 0.9e |
| Q3: To what extent did you make gains in any of the following as a result of what you did in this exercise? | ||
| A. Understanding the relevance of this field to real world issues | 2.9 ± 1.2 | 1.9 ± 0.8d |
| B. Confidence in your ability to do this field | 2.8 ± 1.0 | 2.0 ± 0.9e |
| C. Enthusiasm for subject | 2.8 ± 1.1 | 1.9 ± 0.7d |
aQuestions from evaluations are listed. Students chose a number from 1 to 5, with 1 representing the best result, 3 representing an average result, and 5 representing the worst result. The mean responses ± SD are shown.
bMean responses ± SD from laboratory sections for which the instructor presented the laboratory exercise for the first time. Seventy-six students were surveyed.
cMean responses ± SD from laboratory section for which the instructor presented the laboratory exercise previously. Twenty students were surveyed.
dSignificantly different from course taught by instructor for first time (Student's t test; p < 0.001).
eSignificantly different from course taught by instructor for first time (Student's t test; p < 0.05).
Table 2.
Qualitative assessment of vesicle transport and fusion laboratory experiment
| Knowledge of cell biology topics |
| Q1: How did your observations of GFP-tagged synaptic vesicles increase your understanding of vesicle transport and fusion in neurons? |
| Q2: As a result of this laboratory exercise, what connections did you make between the ability of an organism to function normally and the processes happening inside its cells? |
| Q3: In what ways has this laboratory exercise increased your understanding of model organisms and how they can help us understand cell biology processes in humans? |
| Experiential learning and team work |
| Q4: Explain how designing experiments to understand organism movement and vesicle transport increased your ability to think about cell biology problems. |
| Q5: In what ways did working in a team help you to solve problems in this laboratory exercise? |
| Comparing this exercise to other cell biology exercises |
| Q6: What aspects of this laboratory were more informative to you than other cell biology laboratory exercises? |
Students were given and asked to respond to the questions. Fifty-four students were surveyed.
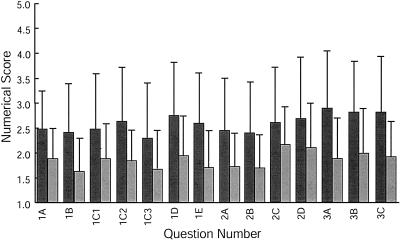
Figure 4.
Quantitative analysis of student response to vesicle transport and fusion exercise. Results from a Likert-style questionnaire are shown. The first bar for each question represents the response from students taught by an instructor who was leading the exercise for the first time. The second bar for each question represents the response from students taught by an instructor who had led the exercise previously. A score of 1 represents the best response, a score of 3 represents an average response, and a score of 5 represents the worst response.
Responses to Likert-Style Questionnaire
Students were asked to respond to several questions addressing how well they learned cell biological concepts. On the numerical scale provided with the questions, a score of “one” represented the best possible answer, “three” represented an average response, and “five” represented the worst possible answer. Students compared their level of learning as a result of this exercise to the other exercises in the course. The questions they addressed were divided into three categories: 1) How much did each of the following aspects of the laboratory exercise help your learning? 2) As a result of your work in this exercise, how well do you think that you now understand each of the following? 3) To what extent did you make gains in any of the following as a result of what you did in this exercise?
Student responses to the Likert-scale questionnaire are separated into two groups. One group represents students in laboratory sections with instructors who were leading the exercise for the first time. The second group represents students in a laboratory section with an instructor who has led the exercise previously. Responses were divided in this manner, because one section was taught by an instructor who ran the laboratory exercise previously, while all other sections were taught by instructors running the laboratory exercise for the first time. Student response to the exercise varied depending on whether the laboratory instructor had taught the exercise previously.
In response to the three categories of questions, students indicated that they improved their knowledge of cell biology topics better than from an average laboratory exercise. Data collected from sections for which instructors taught the exercise for the first time indicated better than average scores for all questions in the three categories. When error was taken into account, however, all scores from these groups of students ranged poorer than average (Figure 4). Data collected from sections with an experienced instructor indicated that this exercise was better than average for all questions when compared with other exercises in the course. All questions in the three categories were also scored better than or equal to average when error was taken into account (Figure 4). Responses to questions specifically addressing learning of the concepts vesicle transport, neurotransmitter release, affect of nervous system on behavior, and GFP were equal to or better than average. According to the results from a Student's t test, p < 0.05 for all questions asked, indicating that there was a significant difference in responses from students in sections taught by inexperienced versus experienced instructors.
Written Responses to Specific Questions About Learning
Students were asked a series of directed questions for which they provided written answers rather than numerical scores. Fifty-four students were asked six questions (Table 2). These questions are divided into three categories: 1) knowledge of cell biology topics, 2) experiential learning and teamwork, and 3) comparing this exercise with other cell biology exercises. Students wrote detailed responses to questions and indicated that the laboratory exercise played a positive role in learning cell biology concepts.
Students indicated that they increased their knowledge of specific cell biology concepts. These concepts included vesicle transport and fusion, the affect of cell function on behavior, and the use of model organisms to understand cell biology processes. Students identified advances they made in understanding vesicle transport and fusion, writing that they “Better understood function/importance of motor proteins and vesicle fusion in neurons” and that “We were able to directly correlate the movement with what was happening inside the cell. We learned about vesicles, their transport, and their binding.” In response to a question about cell function and behavior, students wrote, “It helped us understand the functionality of the vesicle transport system as well as how mutations in such a system affect the organism” and “After this lab exercise, I was able to understand that mutations occurring in the nervous system can drastically affect the muscle function and movement of an organism.” In response to a question designed to assess connections students made between the use of model organisms and an increase in understanding of cell biological topics, a student wrote, “The lab was helpful, because I could apply what I saw in the C. elegans to humans, and understand how some human motor disorders work.”
The second category of questions was designed to assess how an inquiry-based team exercise affected the manner in which students thought about cell biology topics. In response to a question asking how designing experiments helped to increase their ability to think about cell biology problems, a student indicated a direct connection between designing experiments and understanding vesicle transport. This student wrote, “Thinking of ways to observe and identify such problems really made us try to go through the whole process of vesicle transport and understand the significance of all aspects of this process.” Another student considered how designing an experiment allowed connections to be made between movement of a whole organism and the processes inside of its cells: “The designing of this type of experiment allows you to relate concepts of cell biology like vesicle transport to physical problems, you can learn that the vesicles help carry messages, but in an experiment you can observe how that relates to your physical movement and behavior.” Students indicated that working in a team aided the learning process, writing, “The team members combined their knowledge of the process of vesicle fusion at synapses and helped to point out what was being observed. We also collaborated on making an experiment to test why the organisms were damaged. We all had different theories and hypotheses and this helped us think more critically about the mutant worms.”
We next asked how the format of this inquiry-based exercise helped students to increase their knowledge as compared with other laboratory exercises in the course. In response, a student commented on the research aspect of the exercise as well as the use of specific tools. This student wrote, “The experiment related to real scientific research problems, showed the uses of model organisms and also combined work with a new microscope.” Another student discussed a very specific benefit of this laboratory exercise that was provided by the use of GFP, writing, “In other labs, we inferred what was going on within cells or organisms, but this lab actually enabled us to see everything first hand.”
DISCUSSION
The laboratory exercise successfully tied cutting edge research in the laboratory with an undergraduate course, and illustrated ideas that were discussed during the lecture portion of the course (National Research Council, 1997). The concepts explored included vesicle transport and neurotransmitter release, which are carefully studied processes in the field of cell biology. Utilizing the results of professional scholarship to teach biology principles is one way to enhance learning in an undergraduate course (Advisory Committee to the National Science Foundation Directorate for Education and Human Resources, 1996). This goal was achieved by utilizing strains of C. elegans developed in a research laboratory in a course exercise.
Connecting Organismal Behavior with Synaptic Vesicle Transport and Fusion
Students successfully designed two experiments to help them to make connections between function of the nervous system and behavior. Through the use of team discussions, they surmised that nervous system defects would have an adverse effect on movement. They designed simple experiments to analyze movement and categorized strains of worms as having either normal nervous systems or defective nervous systems. This simple analysis, however, was not sufficient to determine the type of nervous system defect in the worms. Through further team discussion, they developed more complex experiments to deduce whether the worms had defects in vesicle transport or fusion in neurons. These experiments were made possible through the use of strains with GFP-tagged synaptic vesicles. Students determined the normal pattern of GFP-tagged vesicles in worms and compared it with the patterns in worms with nervous system defects. Through observations of these patterns, they determined which type of defect was associated with each worm strain. The simple structure of the nervous system in C. elegans and the fact that vesicles were tagged with GFP enabled students in an introductory cell biology course to design and carry out the experiments as a team.
Instructors' Observations of Student Activities
Students were observed to respond well to this laboratory experience. They made connections between the behavior of a whole organism and the cellular processes that control this behavior. The lab was also successful technically; the worms were easy to observe with the stereomicroscope and the method for preparing slides was straightforward. For some students, it took several minutes to find the worms with the fluorescent microscope. The students were less familiar with this larger, more complex microscope than with the compound microscopes they use frequently in the laboratory. Provided there were plenty of worms on the slides, students were able to locate worms easily. Students were very excited to use the fluorescent microscope and to see GFP. During lecture, we discussed both microscopy and fluorescent tags, and the students enjoyed seeing them in action.
Assessment of Exercise
The course assessment showed that the exercise was a successful learning tool. Results from both a quantitative Likert-style questionnaire and qualitative questions indicated that students increased their knowledge of cell biology topics. Specifically, students better understood the concepts of vesicle transport and fusion, the affect of the nervous system on behavior, and the effectiveness of using GFP in their studies. Both types of assessment also indicated that students learned better as a result of the inquiry-based exercise compared with the other exercises in the course. Students indicated that teamwork and experimental design helped them to learn more effectively. They also indicated that GFP was a useful tool in this exercise because direct observations of GFP helped them visualize defects in the nervous system and correlate them with organismal function.
Results from student assessment with a Likert-style questionnaire indicated that students learned better from an experienced instructor. It is not surprising that an experienced instructor guided students though a complex thought exercise more successfully than inexperienced instructors. We addressed the issue in this article so that a person running the exercise for the first time is not discouraged if they do not initially obtain the desired learning results.
Additional Activity
Because some lab groups must wait to work with the fluorescent microscope, an additional activity can be included. The waiting lab groups can observe and draw cells using standard compound microscopes and prepared slides of tissue sections. This activity familiarizes students with the actual appearance of the cells that are often drawn schematically during class. In our laboratory sections, we gave students three different slides and asked them to draw the appearance of individual cells. From a section of skeletal muscle, they were asked to draw a muscle cell, including myosin and the boundaries of a sarcomere. On a slide with a section of gallbladder, students drew epithelial cells, showing microvilli, if visible. The third slide was a spinal cord section and students were asked to draw the structure of a neuron. The exercise helped students to understand that cells with different functions can have very different shapes. It also showed the students the cell bodies and axons of neurons, which are relevant to this study.
Acknowledgments
ACKNOWLEDGMENTS
We thank Mike Nonet for kindly providing us with the GFP strains NM1233 and NM440. Thanks to Tony Lobo, Lynn Mahaffy, and Jim Sidie for using this laboratory exercise in their laboratory sections. Thanks to Rebecca Roberts and Tony Lobo for critical reading of the manuscript, and to Pat Gross for discussions about assessment of learning. This work was supported by funds from the Ursinus College Biological Sciences Research Fund and the Howard Hughes Medical Institute. This material is based on work supported by the National Science Foundation under Grant No. 0092733.
REFERENCES
- Advisory Committee to the National Science Foundation Directorate for Education and Human Resources . Vol. 1 National Science Foundation; Washington, DC: 1996. Shaping the Future: New Expectations for Undergraduate Education in Science, Mathematics, Engineering, and Technology. [Google Scholar]
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. Media preparation and bacteriological tools. In: Benson V., editor. Current Protocols in Molecular Biology. John Wiley & Sons; New York, NY: 1998. pp. 1.1.1–1.1.6. [Google Scholar]
- Bargmann C. I. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282(5396):2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Brose N., Hofmann K., Hafa Y. Mammalian homologues of Caenorhabditis elegans unc-13 gene define a novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Division of Undergraduate Science, Engineering, and Mathematics Education, Directorate for Science and Engineering Education, National Science Foundation . National Science Foundation; Washington, DC: 1989. Report on the National Science Foundation Disciplinary Workshops on Undergraduate Education. Recommendations of the Disciplinary Taskforces Concerning Critical Issue in U.S. Undergraduate Education in the Sciences, Mathematics, and Engineering. [Google Scholar]
- Hall D. H., Hedgecock E. M. Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans . Cell. 1991;65:837–847. doi: 10.1016/0092-8674(91)90391-b. [DOI] [PubMed] [Google Scholar]
- Hall D. M., Russell R. L. The posterior nervous system of the nematode Caenorhabditis elegans: serial reconstruction of identified neurons and complete pattern of synaptic interactions. J Neurosci. 1991;11:1–22. doi: 10.1523/JNEUROSCI.11-01-00001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. W., Johnson R. T., Smith K. A. Active Learning: Cooperation in the College Classroom, Interaction. 1991.
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995;377(6547):351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- McDermott J. B., Aamodt S., Aamodt E. ptl-1, a Caenorhabditis elegans gene whose products are homologous to the tau microtubule-associated proteins. Biochemistry. 1996;35(29):9415–9423. doi: 10.1021/bi952646n. [DOI] [PubMed] [Google Scholar]
- National Research Council. National Academy Press; Washington, DC: 1997. Science Teaching Reconsidered. [Google Scholar]
- Nonet M. L. Visualization of synaptic specializations in live C.elegans with synaptic vesicle protein-GFP fusions. J Neurosci Methods. 1999;89(1):33–40. doi: 10.1016/s0165-0270(99)00031-x. [DOI] [PubMed] [Google Scholar]
- Otsuka A. J., Jeyaprakash A., Garcia-Añoveros J., Tang L. Z., Fisk G., Hartshorne T., Franco R., Born T. The C. elegans unc-104 gene encodes a putative kinesin heavy chain-like protein. Neuron. 1991;6:113–122. doi: 10.1016/0896-6273(91)90126-k. [DOI] [PubMed] [Google Scholar]
- Richmond J. E., Davis W. S., Jorgensen E. M. UNC-13 is required for synaptic vesicle fusion in C. elegans . Nature Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour E. Student Assessement of Learning Gains. 1997. www.wcer.wisc.edu/salgains/instructor/
- Strum-Kenny S. The Boyer Commission on Educating Undergraduates in the Research University, Reinventing Undergraduate Education: A Blueprint for America's Research Universities. 1998. http://notes.cc.sunysb.edu/Pres/boyer.nsf
- Sulston J., Hodgkin J. Methods, In: The Nematode Caenorhabditis elegans . In: Wood W. B., editor. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 587–606. [Google Scholar]
- White J. G., Southgate E., Thomson J. N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans . Phil Trans R Soc Lond B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]