Abstract
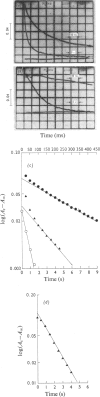
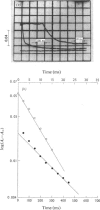
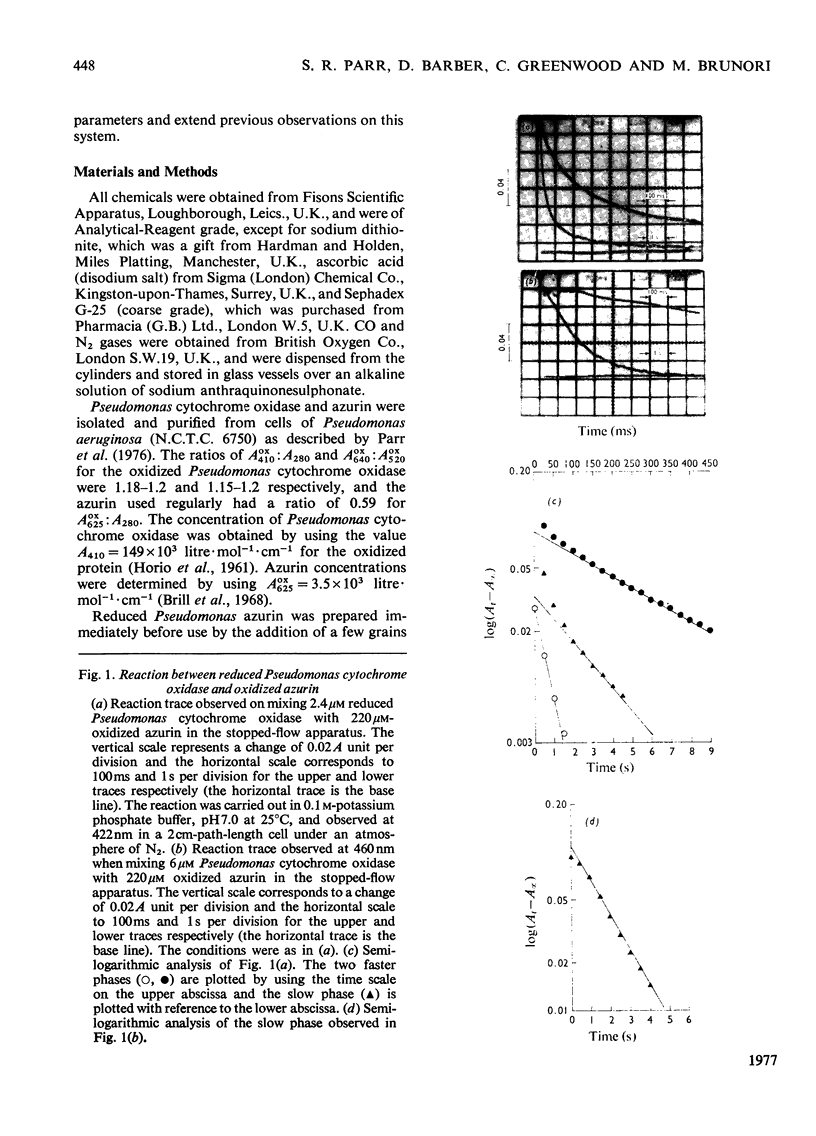
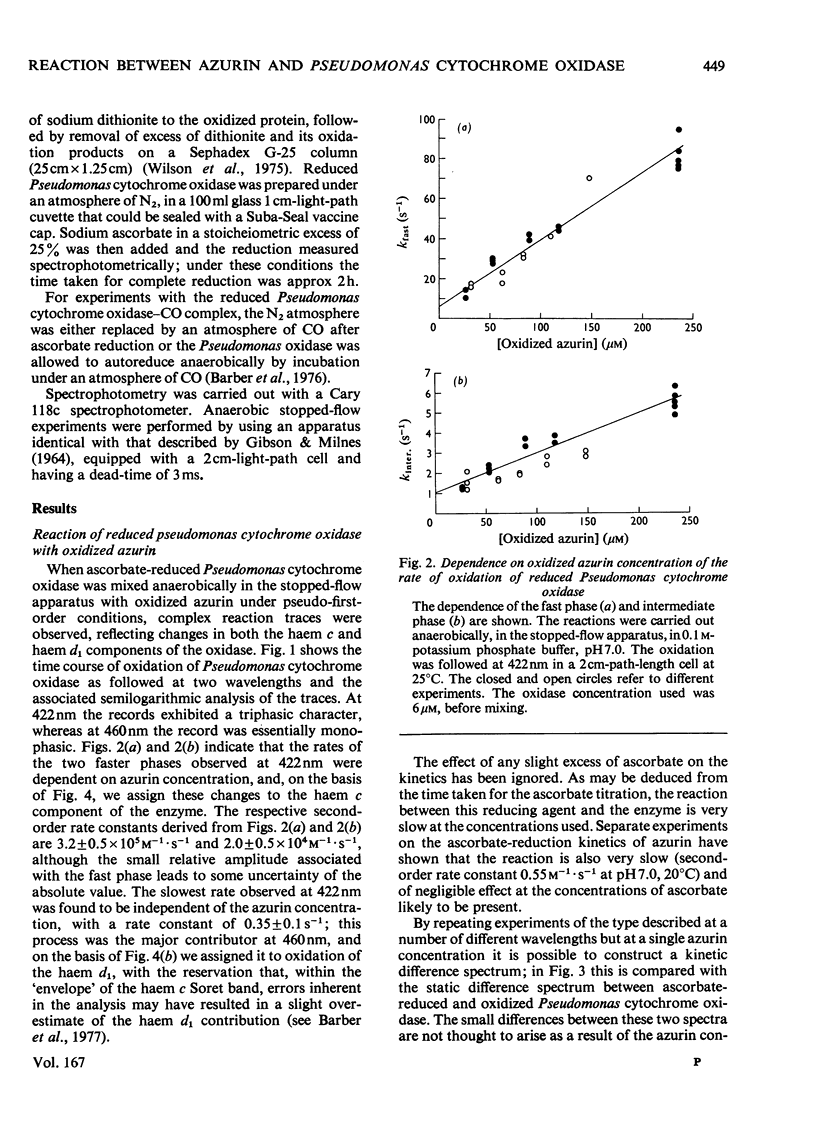
A stopped-flow investigation of the electron-transfer reaction between oxidized azurin and reduced Pseudomonas aeruginosa cytochrome c-551 oxidase and between reduced azurin and oxidized Ps. aeruginosa cytochrome c-551 oxidase was performed. Electrons leave and enter the oxidase molecule via its haem c component, with the oxidation and reduction of the haem d1 occurring by internal electron transfer. The reaction mechanism in both directions is complex. In the direction of oxidase oxidation, two phases assigned on the basis of difference spectra to haem c proceed with rate constants of 3.2 X 10(5)M-1-S-1 and 2.0 X 10(4)M-1-S-1, whereas the haem d1 oxidation occurs at 0.35 +/- 0.1S-1. Addition of CO to the reduced enzyme profoundly modifies the rate of haem c oxidation, with the faster process tending towards a rate limit of 200S-1. Reduction of the oxidase was similarly complex, with a fast haem c phase tending to a rate limit of 120S-1, and a slower phase with a second-order rate of 1.5 X 10(4)M-1-S-1; the internal transfer rate in this direction was o.25 +/- 0.1S-1. These results have been applied to a kinetic model originally developed from temperature-jump studies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber D., Parr S. R., Greenwood C. Some spectral and steady-state kinetic properties of Pseudomonas cytochrome oxidase. Biochem J. 1976 Aug 1;157(2):431–438. doi: 10.1042/bj1570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D., Parr S. R., Greenwood C. The reduction of Pseudomonas cytochrome c551 oxidase by chromous ions. Biochem J. 1977 Jun 1;163(3):629–632. doi: 10.1042/bj1630629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill A. S., Bryce G. F., Maria H. J. Optical and magnetic properties of Pseudomonas azurins. Biochim Biophys Acta. 1968 Feb 19;154(2):342–351. doi: 10.1016/0005-2795(68)90048-2. [DOI] [PubMed] [Google Scholar]
- Brunori M., Greenwood C., Wilson T. A temperature-jump study of the reaction between azurin and cytochrome c-551 from Pseudomonas aeruginosa. Biochem J. 1974 Jan;137(1):113–116. doi: 10.1042/bj1370113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Parr S. R., Greenwood C., Wilson M. T. A temperature-jump study of the reaction between azurin and cytochrome c oxidase from Pseudomonas aeruginosa. Biochem J. 1975 Oct;151(1):185–188. doi: 10.1042/bj1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., MATSUBARA H., KUSAI K., NAKAI M., OKUNUKI K. High purification and properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1958 Aug;29(2):297–302. doi: 10.1016/0006-3002(58)90188-4. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Kuronen T., Ellfolk N. A new purification procedure and molecular properties of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1972 Sep 20;275(3):308–318. doi: 10.1016/0005-2728(72)90212-5. [DOI] [PubMed] [Google Scholar]
- Kuronen T., Saraste M., Ellfork N. The subunit structure of Pseudomonas cytochrome oxidase. Biochim Biophys Acta. 1975 May 30;393(1):48–54. doi: 10.1016/0005-2795(75)90215-9. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Wilson M. T., Greenwood C. The reaction of Pseudomonas aeruginosa cytochrome c oxidase with carbon monoxide. Biochem J. 1975 Oct;151(1):51–59. doi: 10.1042/bj1510051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P., Pecht I. Conformational equilibria accompanying the electron transfer between cytochrome c (P551) and azurin from Pseudomonas aeruginosa. Biochemistry. 1976 Feb 24;15(4):775–786. doi: 10.1021/bi00649a008. [DOI] [PubMed] [Google Scholar]
- Shimada H., Orii Y. Oxidation-reduction behavior of the heme c and heme d moieties of Pseudomonas aeruginosa nitrite reductase and the formation of an oxygenated intermediate at heme d1. J Biochem. 1976 Jul;80(1):135–140. doi: 10.1093/oxfordjournals.jbchem.a131245. [DOI] [PubMed] [Google Scholar]
- Strickland S., Palmer G., Massey V. Determination of dissociation constants and specific rate constants of enzyme-substrate (or protein-ligand) interactions from rapid reaction kinetic data. J Biol Chem. 1975 Jun 10;250(11):4048–4052. [PubMed] [Google Scholar]
- Wharton D. C., Gudat J. C., Gibson Q. H. Cytochrome oxidase from Pseudomonas aeruginosa. I. Reaction with copper protein. Biochim Biophys Acta. 1973 Apr 5;292(3):611–620. doi: 10.1016/0005-2728(73)90009-1. [DOI] [PubMed] [Google Scholar]
- Wilson M. T., Greenwood C., Brunori M., Antonini E. Electron transfer between azurin and cytochrone c-551 from Pseudomonas aeruginosa. Biochem J. 1975 Mar;145(3):449–457. doi: 10.1042/bj1450449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. A nitrite reducing system reconstructed with purified cytochrome components of Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 Oct 28;53:294–308. doi: 10.1016/0006-3002(61)90442-5. [DOI] [PubMed] [Google Scholar]