Abstract
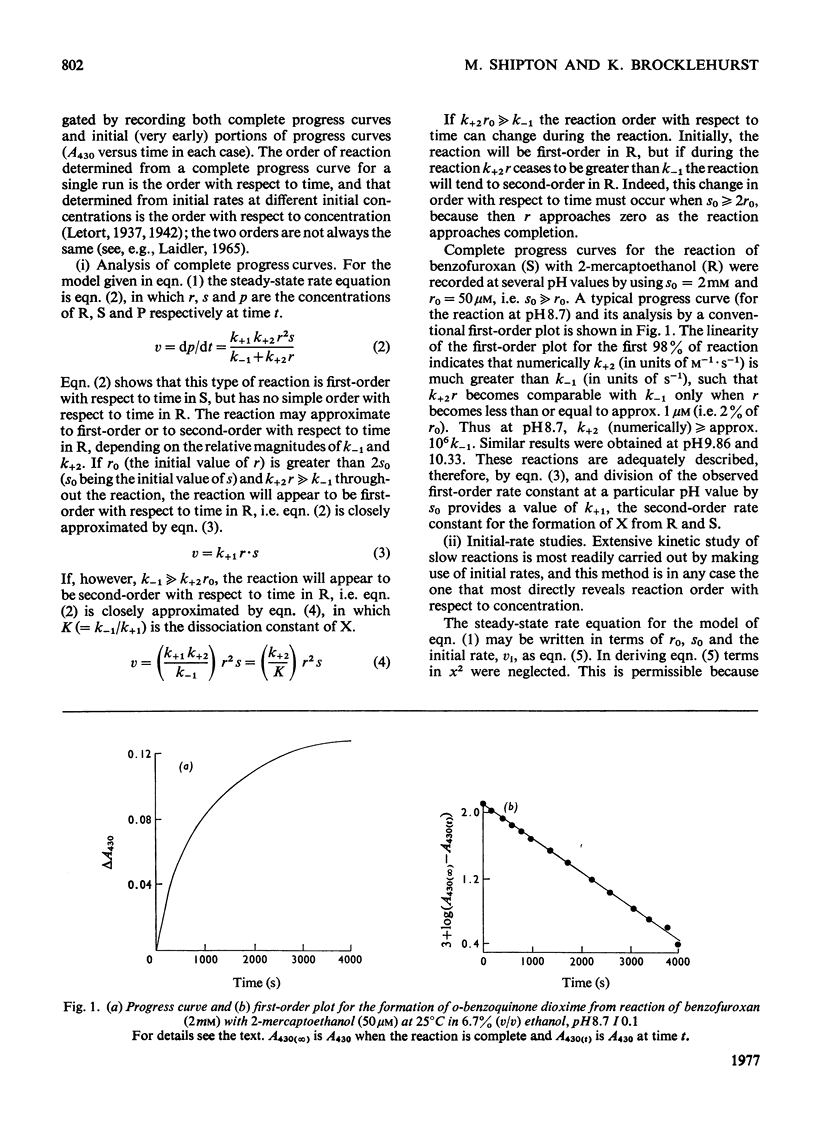
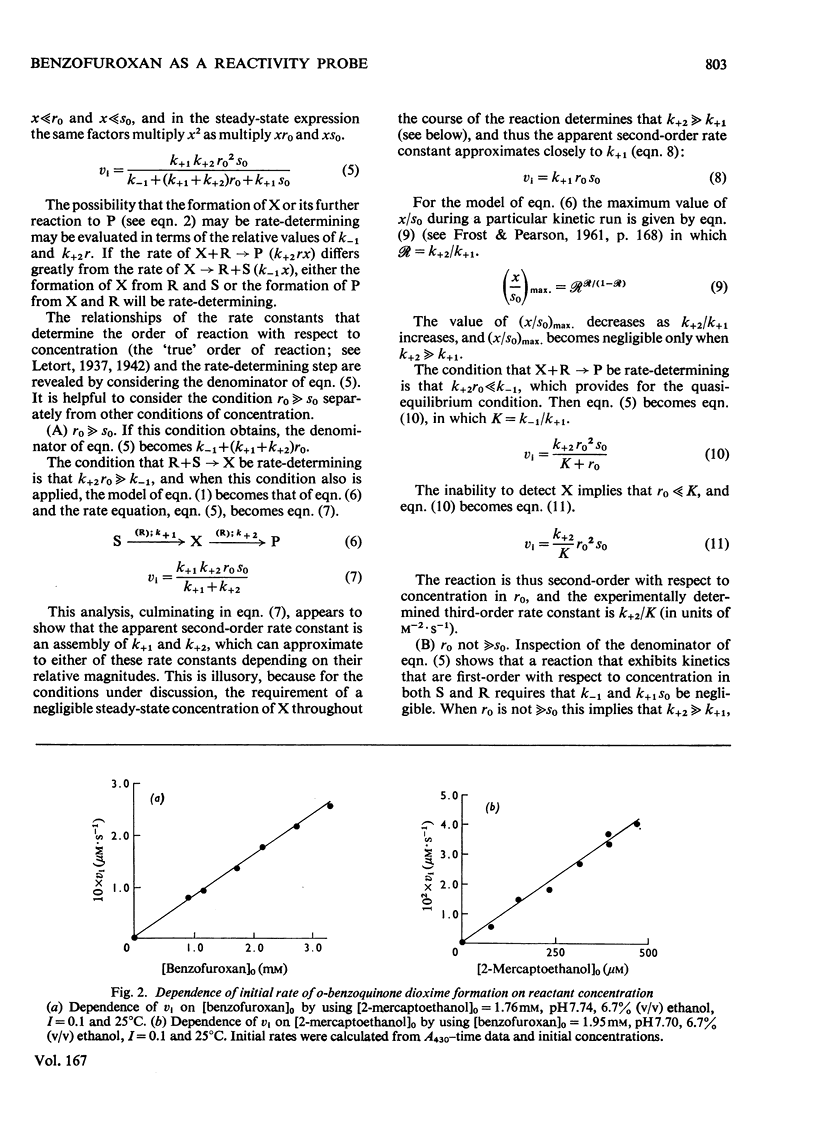
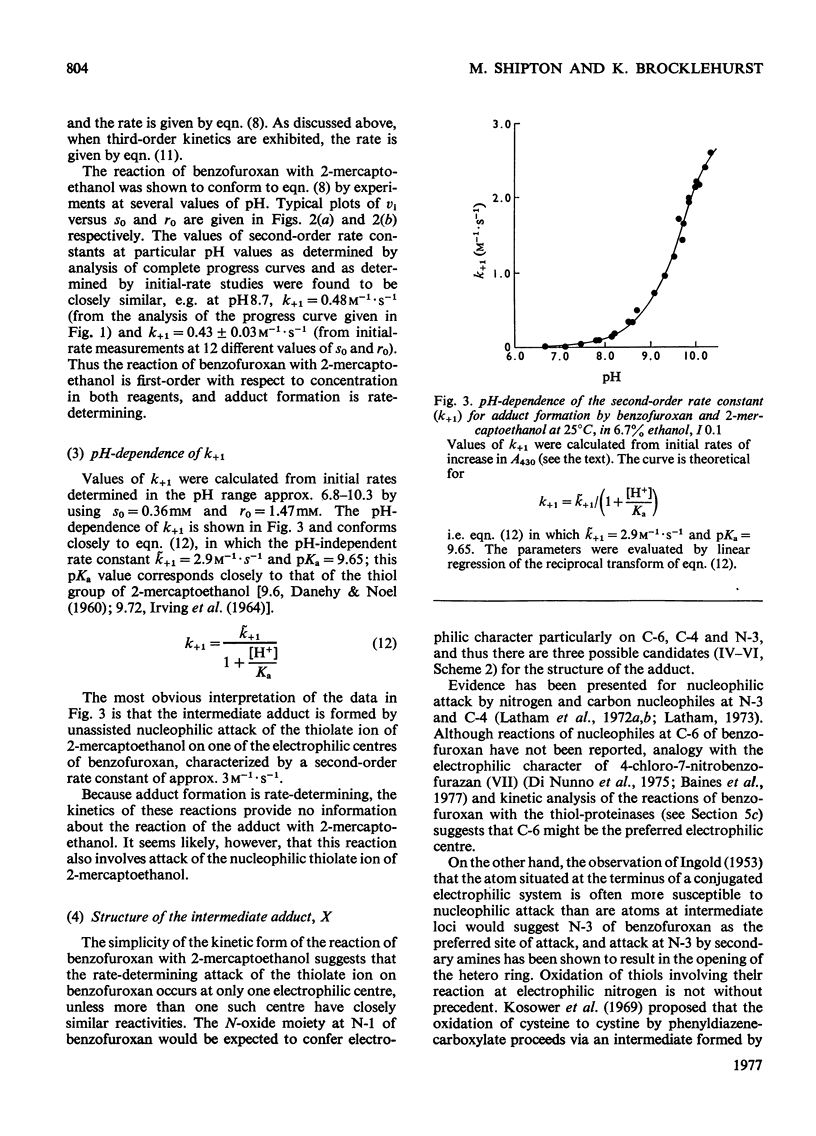
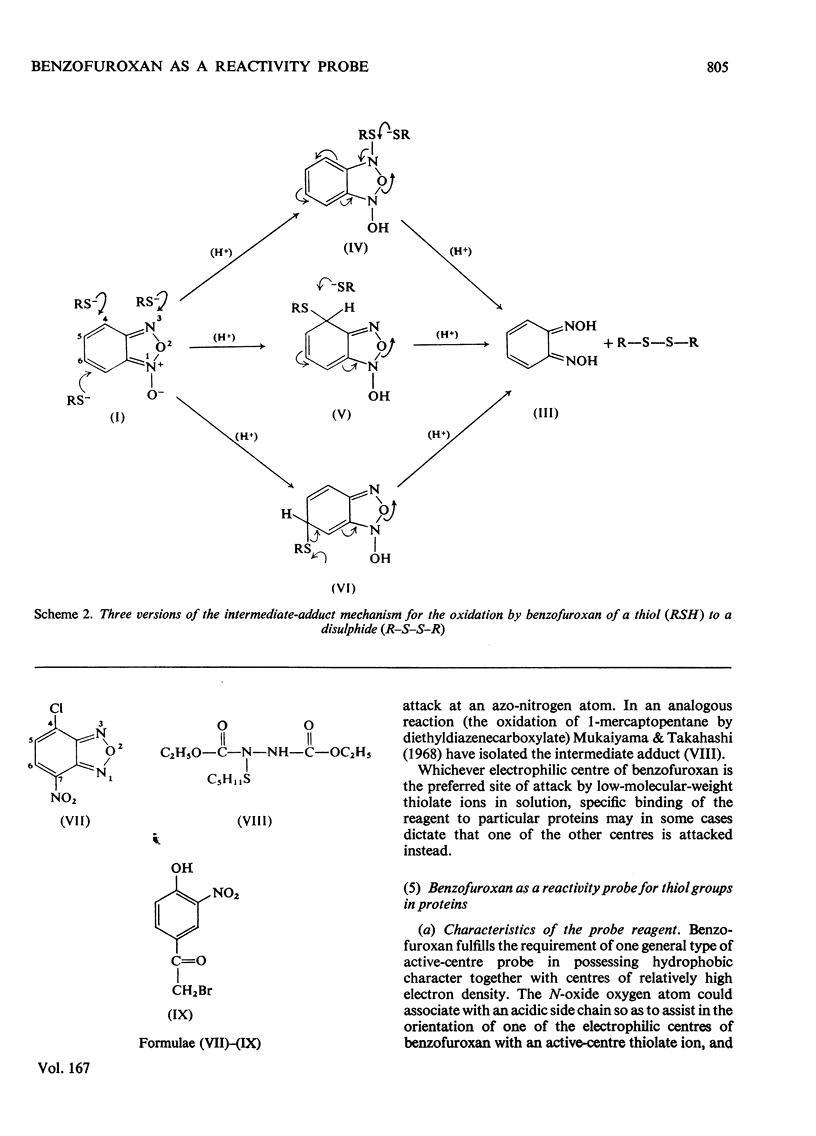
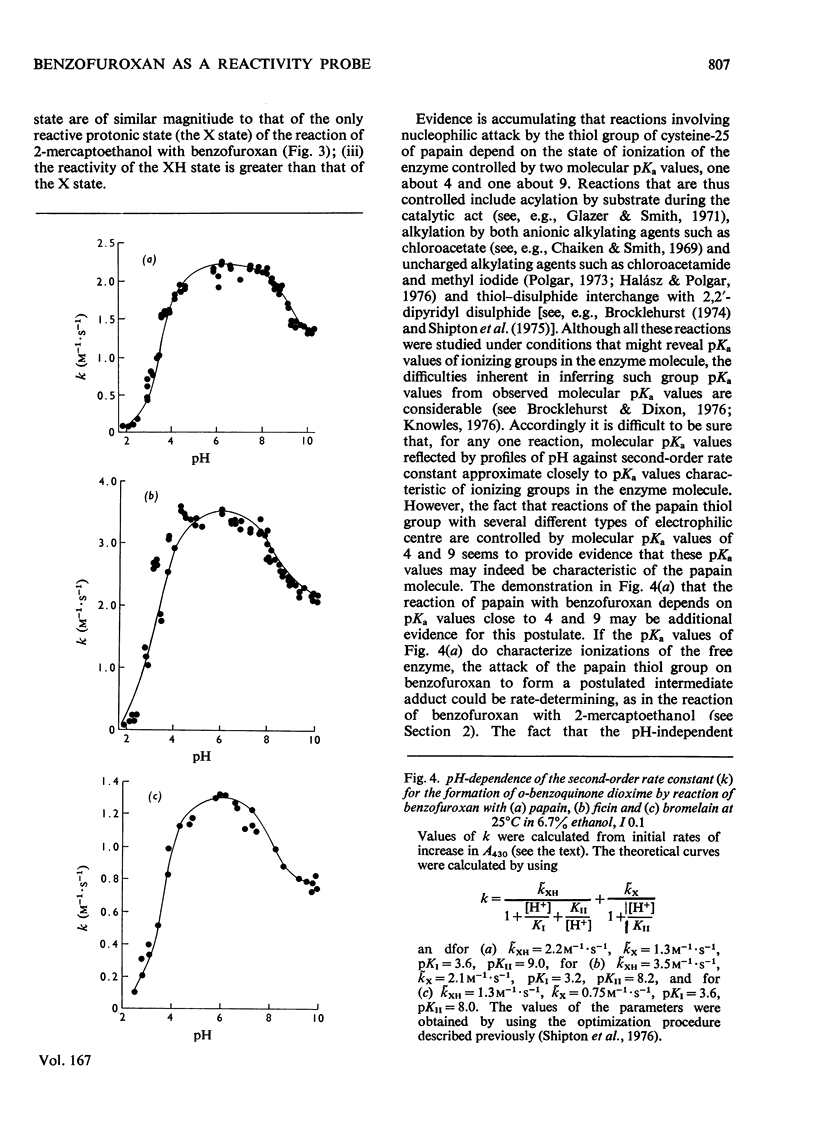
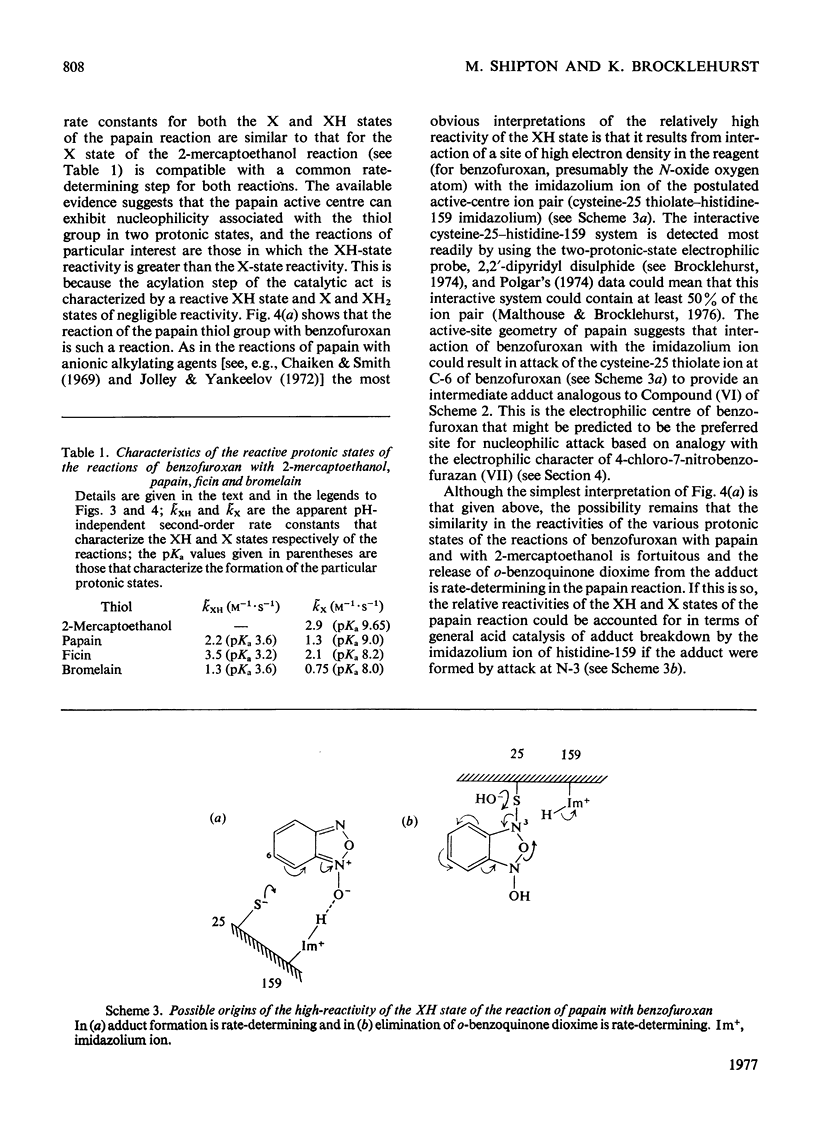
1. The characteristics of benzofuroxan (benzofurazan 1-oxide, benzo-2-oxa-1,3-diazole N-oxide) that relate to its application as a reactivity probe for the study of environments of thiol groups are discussed. 2. To establish a kinetic and mechanistic basis for its use as a probe, a kinetic study of its reaction with 2-mercaptoethanol was carried out. 3. This reaction appears to proceed by a rate-determining attack of the thiolate ion on one of the electrophilic centres of benzofuroxan (possibly C-6) to provide a low steady-state concentration of an intermediate adduct; rapid reaction of this adduct with a second molecule of thiol gives the disulphide and o-benzoquinone dioxime. 4. The effects of the different types of environment that proteins can provide on the kinetic characteristics of reactions of thiol groups with benzofuroxan are delineated. 5. Benzofuroxan was used as a thiolspecific reactivity probe to investigate the active centres of papain (EC 3.4.22.2), ficin (EC 3.4.22.3) and bromelain (EC 3.4.22.4). The results support the concept that the active centres of all three enzymes either contain a nucleophilic thiolate ion whose formation is characterized by a pKa of 3-4 and whose reaction with an electrophile can be assisted by interaction of a site of high electron density in the electrophile with active-centre imidazolium ion of pKa 8-9, or can provide such ions by protonic redistribution in enzyme-reagent or enzyme-substrate complexes.
Full text
PDF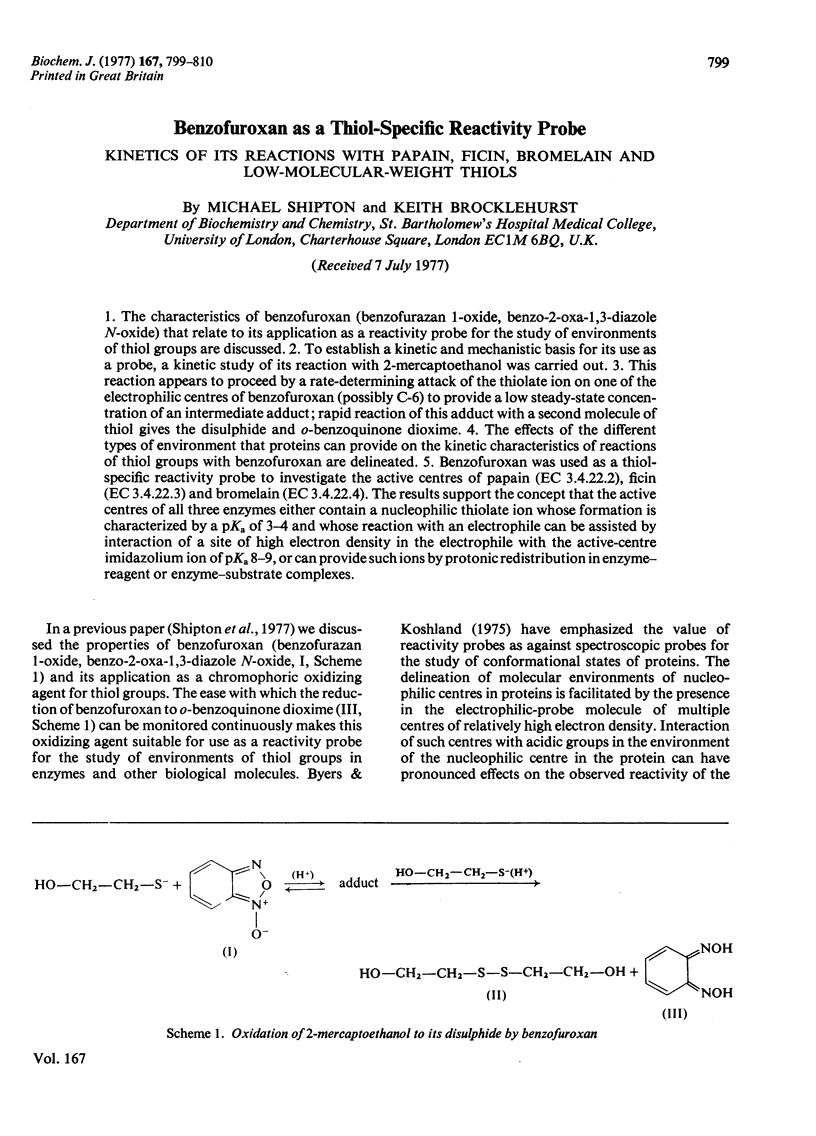





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines B. S., Allen G., Brocklehurst K. The highly electrophilic character of 4-chloro-7-nitrobenzofurazan and possible consequences for its application as a protein-labelling reagent. Biochem J. 1977 Apr 1;163(1):189–192. doi: 10.1042/bj1630189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocklehurst K., Dixon H. B. PH-dependence of the steady-state rate of a two-step enzymic reaction. Biochem J. 1976 Apr 1;155(1):61–70. doi: 10.1042/bj1550061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers L. D., Koshland D. E., Jr The specificity of induced conformational changes. The case of yeast glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1975 Aug 12;14(16):3661–3669. doi: 10.1021/bi00687a023. [DOI] [PubMed] [Google Scholar]
- Chaiken I. M., Smith E. L. Reaction of the sulfhydryl group of papain with chloroacetic acid. J Biol Chem. 1969 Oct 10;244(19):5095–5099. [PubMed] [Google Scholar]
- Dixon H. B. The unreliability of estimates of group dissociation constants. Biochem J. 1976 Mar 1;153(3):627–629. doi: 10.1042/bj1530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halász P., Polgár L. Effect of the immediate environment on the reactivity of the essential -SH group of papain. Eur J Biochem. 1976 Dec 11;71(2):571–575. doi: 10.1111/j.1432-1033.1976.tb11147.x. [DOI] [PubMed] [Google Scholar]
- Jolley C. J., Yankeelov J. A., Jr Reaction of papain with -bromo- -(5-imidazolyl)propionic acid. Biochemistry. 1972 Jan 18;11(2):164–169. doi: 10.1021/bi00752a005. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. The intrinsic pKa-values of functional groups in enzymes: improper deductions from the pH-dependence of steady-state parameters. CRC Crit Rev Biochem. 1976 Nov;4(2):165–173. doi: 10.3109/10409237609105457. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Song K. R., Kosower E. M., Correa W. Glutathione. II. Chemical aspects of azoester procedure for oxidation to disulfide. Biochim Biophys Acta. 1969 Oct 7;192(1):8–14. doi: 10.1016/0304-4165(69)90003-8. [DOI] [PubMed] [Google Scholar]
- Malthouse J. P., Brocklehurst K. Preparation of fully active ficin from Ficus glabrata by covalent chromatography and characterization of its active centre by using 2,2'-depyridyl disulphide as a reactivity probe. Biochem J. 1976 Nov;159(2):221–234. doi: 10.1042/bj1590221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris R., Brocklehurst K. A convenient method of preparation of high-activity urease from Canavalia ensiformis by covalent chromatography and an investigation of its thiol groups with 2,2'-dipyridyl disulphide as a thiol titrant and reactivity probe. Biochem J. 1976 Nov;159(2):245–257. doi: 10.1042/bj1590245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polgár L. Mercaptide-imidazolium ion-pair: the reactive nucleophile in papain catalysis. FEBS Lett. 1974 Oct 1;47(1):15–18. doi: 10.1016/0014-5793(74)80415-1. [DOI] [PubMed] [Google Scholar]
- Polgár L. On the mode of activation of the catalytically essential sulfhydryl group of papain. Eur J Biochem. 1973 Feb 15;33(1):104–109. doi: 10.1111/j.1432-1033.1973.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Purdie J. W. Gamma radiolysis of cystine in aqueous solution. Dose-rate effects and a proposed mechanism. J Am Chem Soc. 1967 Jan 18;89(2):226–228. doi: 10.1021/ja00978a007. [DOI] [PubMed] [Google Scholar]
- Shipton M., Kierstan M. P., Malthouse J. P., Stuchbury T., Brocklehurst K. The case for assigning a value of approximately 4 to pKa-i of the essential histidine-cysteine interactive systems of papain, bromelain and ficin. FEBS Lett. 1975 Feb 15;50(3):365–368. doi: 10.1016/0014-5793(75)80529-1. [DOI] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. 4-Chloro-7-nitrobenzo-2-oxa-1,3-diazole as a reactivity probe for the investigation of the thiol proteinases. evidence that ficin and bromelain may lack carboxyl groups conformationally equivalent to that of aspartic acid-158 of papain. Biochem J. 1976 Nov;159(2):235–244. doi: 10.1042/bj1590235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipton M., Stuchbury T., Brocklehurst K. Evaluation of benzofuroxan as a chromophoric oxidizing agent for thiol groups by using its reactions with papain, ficin, bromelain and low-molecular-weight thiols. Biochem J. 1977 Mar 1;161(3):627–637. doi: 10.1042/bj1610627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenfels K., Eisele B. Stereospecific alkylation with asymmetric reagents. Eur J Biochem. 1968 Jan;3(3):267–275. doi: 10.1111/j.1432-1033.1968.tb19526.x. [DOI] [PubMed] [Google Scholar]
- Wassarman P. M., Major J. P. The reactivity of the sulfhydryl groups of lobster muscle glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1969 Mar;8(3):1076–1082. doi: 10.1021/bi00831a039. [DOI] [PubMed] [Google Scholar]


