Abstract
Objectives
To investigate the influence of MEK5/ERK5 pathway on mitophagy in osteosarcoma (OS), as well as the involved molecular mechanisms.
Methods
The overlapped genes of mitophagy-related genes from MSigDB database and DEGs between metastatic and primary OS groups from GSE32981 were identified. GSVA of mitophagy-related pathways between the metastatic and primary groups were analyzed. The relationships between Nur77 and mitophagy-related pathways, prognosis, immune infiltrating cells, immune response gene sets were investigated. Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and western blotting were utilized to assess the expression levels of MEK5, ERK5, Nur77, PINK1, and Parkin. Cellular behaviors and mitochondrial potential were evaluated via CCK-8, Transwell assay and JC-1 staining.
Results
Total 4 overlapped genes were obtained as mitophagy-related DEGs, including GABARAPL1, HIF1A, PINK1, and RB1CC1. The activity scores of 3 mitophagy-related pathways exhibited significant differences between metastatic and primary groups. Importantly, Nur77 was significantly negatively correlated with a mitophagy-related pathway (GOBP MITOPHAGY: R = − 0.48, P = 0.02). The Nur77 expression in metastatic group was remarkedly higher than that in the primary group (P < 0.001). Patients with high Nur77 expression had poor prognosis, with AUC values all above 0.615 in predicting 1-, 3-, and 5-year survival. In addition, Nur77 was closely related to numerous immune cells, including activated dendritic cells, activated mast cells and M0 macrophages, and immune response gene sets chemokines and cytokines (all P < 0.05). In addition, MEK5/ERK5 pathway is activated in OS, and Nur77 is overexpressed in OS, and MEK5/ERK pathway promotes Nur77 expression, tumorigenesis and mitochondrial function in U2OS cells. Cytosporone B implement significantly increased the tumorigenesis of U2OS cells in sh-MEK5 group, and inhibited the weaken in mitochondrial membrane potential caused by MEK5 downregulation, and reversed the protein levels of mitophagy markers PINK1 and Parkin in sh-MEK5 group.
Conclusions
MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of OS cells. These findings offered promising therapeutic targets for OS.
Keywords: Osteosarcoma, Mitophagy, MEK5–ERK5 pathway, Nur77
Introduction
Osteosarcoma (OS), the commonest malignant bone tumor, is characterized by osteolytic bone destruction and primarily affects adolescents and young adults [1, 2]. Approximately 75% of patients are diagnosed between the ages of 15 and 25, with an annual incidence rate of about 2–3 cases per million [3]. OS is highly malignant, with a propensity for early pulmonary metastasis, resulting in high mortality rates and poor prognosis [4]. With advancements in diagnostic and therapeutic approaches, the 5-year disease-free survival (DFS) rate for OS patients who receive neoadjuvant chemotherapy and surgery can reach 50–70% [5]. However, for patients with pulmonary metastases at the time of initial diagnosis, the 2-year DFS rate is only 28% when there are ≥ 3 metastatic lesions [6]. Approximately 30% of OS patients without metastasis at diagnosis will experience recurrence [7], while about 80% of those with metastasis will face recurrence [8]. Therefore, the identification of diagnostic markers and targets for invasion and metastasis detection is of paramount importance for the effective diagnosis and treatment of OS.
Mitochondria are the main place of cellular oxidative phosphorylation, and ATP generated by mitochondria is the main energy source of cell life activities. However, when mitochondria are damaged, they can lead to the release of reactive oxygen species or apoptosis factors, resulting in cell damage or apoptosis [9, 10]. Mitophagy is a special form of autophagy that is used to identify and remove damaged mitochondria in a timely manner [11]. It has been reported that mitophagy is directly or indirectly related to tumor pathogenesis and has the controlling effect on tumor cells, including OS [12]. Besides, the MEK5–ERK5 signaling pathway, a crucial component of the MAPK cascade, has garnered significant attention owing to its regulatory role in numerous cellular processes, containing proliferation, differentiation, and survival [13]. The MEK5/ERK5 signaling pathway is acknowledged for its pivotal involvement in multiple cancer characteristics and serves as a conduit through which various oncogenes exert their influence [14]. Previous study revealed that MEK5 pathway deregulation implicated the pathogenesis of metastatic prostate cancer, colon cancer, and aggressive OS [15]. Importantly, previous studies revealed that MEK5/ERK5 pathway plays significant roles in mitophagy [16, 17]. Besides, it’s reported that Nur77 is dysregulated when activated ERK5 signaling pathway [18]. In addition, Nur77 could translocate from the nucleus to mitochondria to modulate mitochondrial activities [19]. However, the effect of MEK5/ERK5 pathway on mitophagy in OS, as well as the involved molecular mechanisms are not reported.
This study aims to explore the influence of MEK5/ERK5 pathway on mitophagy in OS, as well as the involved molecular mechanisms. This study may offer valuable understanding to the development of targeted therapeutic strategies for OS.
Methods
Bioinformatic analysis
Mitophagy-related pathways were retrieved from the MSigDB database [20], then mitophagy-related genes were obtained. GSE32981 data set, including 11 metastatic OS samples and 12 primary OS samples, were downloaded from GEO database [21]. Probes were annotated based on the gene chip platform. Subsequently, differentially expressed genes (DEGs) were acquired utilizing the “limma” package (v 3.58.1) [22] with the threshold of P < 0.05 and |log2FC|> 0.5. Then the DEGs were intersected with the obtained mitophagy-related genes, and the overlapped genes were obtained as mitophagy-related DEGs. The expression of these mitophagy-related DEGs in the metastatic and primary OS groups was assessed using the t test method. In addition, GSVA analysis was conducted on the obtained mitophagy-related pathways using “ssGSEA” [23]. This activity scores of the mitophagy-related pathway in different samples were compared. The correlation between Nur77 and pathways were analyzed using Pearson method. Besides, the gene expression profile and clinical data of OS patients were searched from the Target database. Then according to the median value of Nur77 expression, the samples were categorized into high- and low-expression groups. Then the survival analysis was performed using “survival” package (v 3.5–8) [24]. Furthermore, the “CIBERSORT” package (v 0.1.0) [25] was employed to explore the fraction of 22 immune cells in OS samples, and the correlation between Nur77 and immune cells were explored using Spearman correlation method. In addition, the immune response gene sets were downloaded from ImmPort database, and “GSVA” package (v 3.50.1) [26] was applied to calculate the enrichment score of immune related genes in each sample based on GSE32981 data set, then the differences in immune response gene set activity between primary and metastatic OS samples were evaluated using rank sum test. The correlation between the NUR77 and the immune response gene sets was investigated using Spearman correlation method.
Cell culture and transfection
The human normal osteoblast cell line hFOB1.19 (CL-233 h) and OS cell lines MG-63 (CL-157 h) and U2OS (CL-655 h) were sourced from Wuhan SAIOS Biotechnology Co., Ltd. (Wuhan, China). These cell lines were grown in DMEM (C11885500BT, Gibco, CA, USA) with conventional cultivation conditions. To investigate the role of MEK5–ERK5 pathway in OS, the lentiviral vector of MEK5 (sh-MEK5) was constructed to interfer MEK5 expression. shRNA targeting human MEK5 (sh-MEK5, GAGAACCAGGTGCTGGTAATT) was designed at VectorBuilder website. The sh-MEK5 and its negative control (NC) were integrated into pLVX–shRNA2-puro vector to construct their lentiviral vectors. The titer of sh-MEK5 lentivirus was measured to be 1 × 108 TU/mL, with a multiplicity of infection (MOI) of 10. U2OS cells were inoculated into a 96-well plate, with 1 × 104 cells per well, and cultured until the cell density reach 40–60%. Cells were then infected with sh-MEK5 lentivirus. After 3–4 days of transfection, a fluorescent microscope was used to observe the cells. U2OS cells were categorized into five group, containing Control, sh-NC, sh-MEK5, sh-MEK5 + LM22B-10, and sh-MEK5 + Cytosporone B groups. Cells in sh-NC and sh-MEK5 groups were transfected with sh-NC and sh-MEK5 lentiviral vector, respectively. Cells in sh-MEK5 + LM22B-10 group were treated with the 50 μM LM22B-10 (ERK pathway activator) for 48 h based on sh-MEK5 group, and cells in sh-MEK5 + Cytosporone B group were treated with 5 µM Cytosporone B (Nur77 activator) for 30 min based on sh-MEK5 group.
Western blotting
Proteins in cells were extracted for western blotting as described previously [27]. Then the collected proteins were analyzed by SDS–PAGE and transferred to PVDF membrane (FFP24, Beyotime, China). Membranes were incubated with primary antibodies against MEK5 (1:500; #ab210748, Abcam, CB, UK), ERK5 (1:10,000; #ab40809, Abcam, CB, UK), p-ERK5 (1:1000, #AF8146, Affinity, MA, USA), Nur77 (1:500; #ab153914, Abcam, CB, UK), PINK1 (1:1000, #ab216144, Abcam, CB, UK), Parkin (1:1000; #ab73015, Abcam, CB, UK), and GAPDH (1:10,000; #ab181602, Abcam, CB, UK) at 4 °C overnight, followed by incubation with secondary antibody Goat Anti-Rabbit IgG H&L (HRP; 1:2,000; #ab6721, Abcam, CB, UK). Protein band intensities were visualized utilizing an electrochemical luminescence (ECL) reagents (P1000, APPLYGEN, Beijing, China).
Transwell assay
Using Transwell assay, cell migration and invasion were analyzed. Cells in logarithmic growth phase were collected, and trypsin was employed to digest, and then resuspend them in serum-free culture medium and adjusted the cell density to 1 × 106/mL. Then 200 cells were inoculated on Transwell chambers under 5% CO2 at 37 °C, and complete culture medium was added in the lower chamber for 24 h. Then cells that migrated or invaded into the outer chamber were subjected to 0.1% crystal violet staining for 20 min. Three random fields per group were counted via an inverted microscope (DMi3000 B; Leica, Wetzlar, Germany).
Cell counting kit-8 (CCK-8)
Cell viability was assessed through the CCK-8 kit (C0037, Beyotime, China). Initially, 100 µL cell suspension including 2 × 103 cells per was grew in a 96-well plate at 37 °C with 5% CO2. After the cells had fully adhered to the culture dish, they were treated with 50 μM LM22B-10 for 48 h according to the aforementioned grouping. After transfection process, CCK-8 reaction solution (10 µL) was meticulously introduced into each well. Upon completion of the incubation, the absorbance of the samples was quantified at 450 nm utilizing a microplate reader (Wuxi Hiwell Diatek, Jiangsu, Nanjing).
Mitochondrial membrane potential (MMP) assay
The MMP (ΔΨm) of cells were evaluated utilizing the JC-1 staining kit (C2006, Beyotime, China). U2OS cells were plated in a 6-well plate with the density of 3–5 × 105 cells per well. JC-1 dye was added to each well for 20 min. Fluorescent was measured at 525/595 nm via CytoFLEX S (Beckman Coulter Life Sciences, Indianapolis, IN, USA) after washing with JC-1 staining buffer.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Total RNA was extracted using the Trizol reagent (#15596018, Invitrogen, USA). qRT‑PCR was performed using the FastKing RT SuperMix (#KR118; TIANGEN, China) and SYBR Green PCR Master (#A4004M, Lifeint, China). The primer sequences were as follows: human MEK5 forward, 5′‑GCCATTGGAGTTGGACTGGA‑3′ and reverse, 5′‑TGGAGGGCTTCACGTCTCTA‑3'; human ERK5 forward, 5′‑CAAACGAGCGGAGGGAAGAT‑3′ and reverse, 5′‑TTGTGTCCGCCATTTGCTTG‑3′; human Nur77 forward, 5′‑TTGTGTCCGCCATTTGCTTG‑3′ and reverse, 5′‑GATACAGGGCATCTCCGGC‑3′; human GAPDH forward, 5′‑TGTGGGCATCAATGGATTTGG‑3′ and reverse, 5′‑ACACCATGTATTCCGGGTCAAT‑3′. Relative quantities of MEK5, ERK5 and Nur77 mRNA were normalized to GAPDH.
Statistical analysis
The data was showed as the mean ± SD. GraphPad 8.0 software was utilized for all statistical analyses. One-way analysis of variance (ANOVA) was utilized to assess differences between group, followed by Tukey’s post hoc test for comparison. Significance was defined as P < 0.05, denoting statistical significance.
Results
Nur77 is significantly overexpressed and closely related to mitophagy and immune in OS
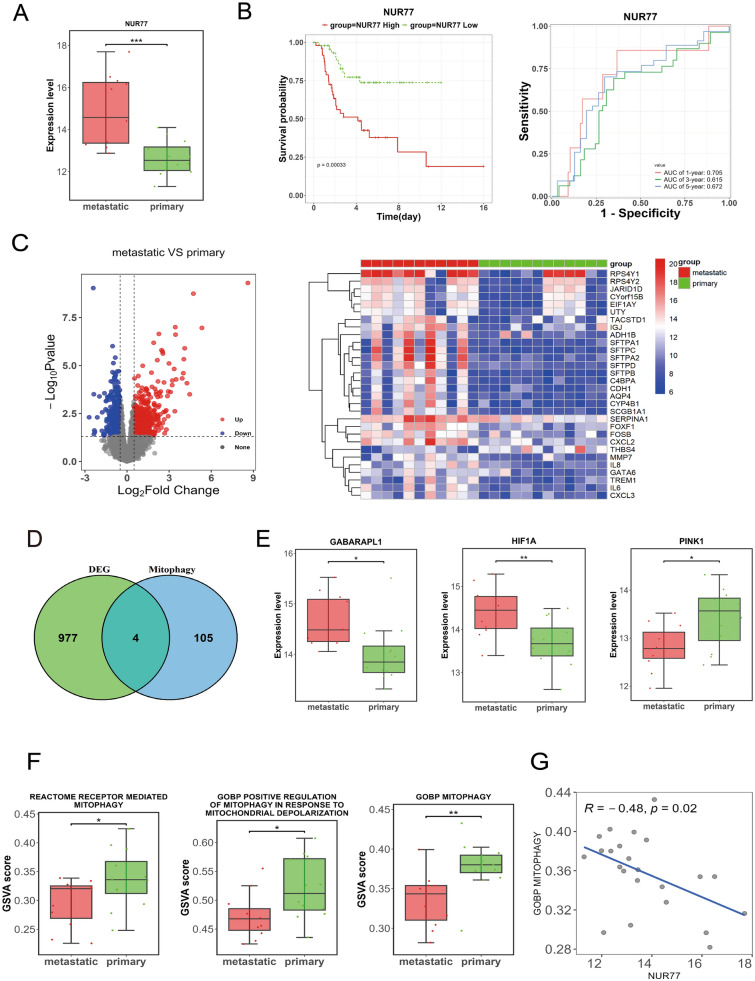
It’s reported that Nur77 is closely associated with OS [28], and closely related to mitophagy [29]. Thus, the correlation between Nur77 and mitophagy was explore in OS. The Nur77 expression in the metastatic group was remarkedly higher than that in the primary group (P < 0.001; Fig. 1A). Moreover, patients in high Nur77 expression group had lower survival rate than those in low expression group (P = 0.00033), with AUC values all above 0.615 in predicting 1-, 3-, and 5-year survival (Fig. 1B). Besides, total 13 mitophagy-related pathways were obtained from MSigDB database, then 109 mitophagy-related genes were also acquired. In addition, total 981 DEGs were obtained, containing 442 up-regulated and 539 down-regulated DEGs (Fig. 1C), and 4 overlapped genes were obtained as mitophagy-related DEGs, containing GABARAPL1, HIF1A, PINK1, RB1CC1 (Fig. 1D). In addition, the expression levels of HIF1A, and GABARAPL1 were remarkedly elevated in metastatic group compared to the primary group (P < 0.05), and the opposite result was found on PINK1 expression (Fig. 1E). Furthermore, GSVA shown that the activity scores of 3 mitophagy-related pathways exhibited significant differences between metastatic and primary groups (all P < 0.05; Fig. 1F). Importantly, Nur77 was significantly negatively correlated with a mitophagy-related pathway (GOBP MITOPHAGY: R = − 0.48, P = 0.02; Fig. 1G). Besides, Fig. 2A, B reveals that the fraction of 4 immune cells existed significant difference between metastatic and primary OS samples (all P < 0.01), and Nur77 was strongly related to numerous immune cells, including activated dendritic cells, activated mast cells and M0 macrophages (Fig. 2C). Furthermore, the differences in immune response gene set activity between primary and metastatic OS samples were evaluated, and antimicrobials, chemokines and cytokines showed significant difference between primary and metastatic OS samples (all P < 0.05; Fig. 2D), and Nur77 was also closely related chemokines and cytokines (Fig. 2E). Therefore, Nur77 was significantly overexpressed and closely related to mitophagy and immune in OS.
Fig. 1.
Nur77 is significantly overexpressed and closely related to mitophagy in osteosarcoma (OS). A Expression level of Nur77 in the metastatic and primary groups. B Kaplan–Meier (KM) curve (left) and receiver operating characteristic (ROC) curve (right) of Nur77. C Volcano map (left) and heatmap (right) of differentially expressed genes (DEGs). D Venn diagram of the intersection of DEGs and mitophagy-related genes. E Expression level of 4 mitophagy-related DEGs in metastatic and primary groups. F Gene set variation analysis (GSVA) scores of three mitophagy-related pathways in the metastatic and the primary group. G Scatter plot of the correlation between Nur77 and the mitophagy-related pathways. *P < 0.05, **P < 0.01, ***P < 0.001
Fig. 2.
Relationship between Nur77 and immune in osteosarcoma (OS). A Immune cell content in each OS sample. B Differences of fraction of immune cells between metastatic and primary OS samples. C Correlation between Nur77 and immune cells. D Differences in immune response gene set activity between primary and metastatic OS samples. E Correlation between Nur77 and immune response gene sets. *P < 0.05, **P < 0.01, ***P < 0.001
MEK5/ERK5 pathway is activated in OS
To investigate whether MEK5/ERK5 pathway involved in development of OS, the expression levels of MEK5 and ERK5 in OS cell lines U2OS and MG63 were explored. In comparison with normal osteoblast cell line hFOB1.19, an obvious upregulation in the mRNA expression of MEK5 and ERK5 in the OS cell lines (U2OS and MG-63) were observed (all P < 0.05, Fig. 3A), as well as the protein expression level (Fig. 3B). In addition, the mRNA expression of Nur77 in OS cell lines (U2OS and MG-63) was significantly increased relative to normal osteoblast cell line hFOB1.19 (all P < 0.05, Fig. 3A). Due to the levels of MEK5, ERK5, and Nur77 in U2OS cells were notably higher than those in MG-63 cells, thus, subsequent research was focus on studying U2OS cells. This data implied that the MEK5/ERK5 pathway was activated and Nur77 was overexpressed in OS, which could be key factors in the progression of OS.
Fig. 3.
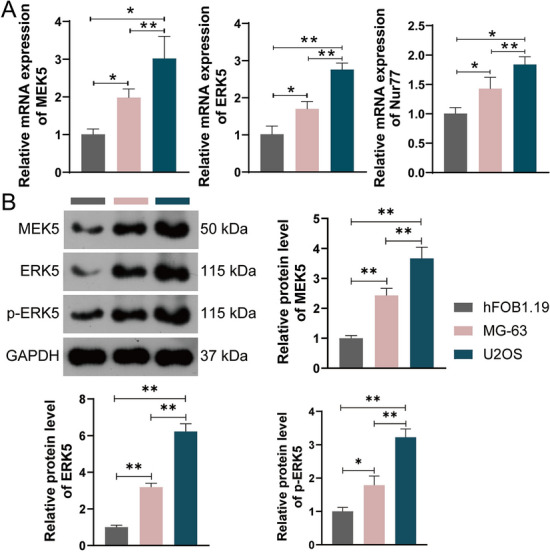
MEK5, ERK5, and Nur77 expression levels in human normal osteoblast cell line hFOB1.19 and osteosarcoma (OS) cell lines U2OS and MG63. A mRNA expression levels. B Protein expression levels. *P < 0.05, **P < 0.01
MEK5/ERK pathway promotes Nur77 expression, tumorigenesis and mitochondrial function in U2OS cells
Accumulating evidences have revealed that MEK5/ERK5 pathway plays a crucial role in several cancer [30, 31]. To clarify the important role of MEK5/ERK pathway in OS, the lentiviral vector of MEK5 (sh-MEK5) was constructed and transfected in U2OS cells to interfer MEK5 expression, and the transfection efficiency were measured (Fig. 4A, B). In addition, previous study has revealed that the expression of Nur77 is up-regulated when activated ERK5 signaling pathway [18]. Thus, it is speculated that Nur77 is up-regulated when activated MEK5/ERK pathway in OS. As illustrated in Fig. 4B, the Nur77 expression was remarkedly suppressed in U2OS cells after MEK5 downregulation (P < 0.01), while this alteration was reversed after LM22B-10 (ERK pathway activator) implement. Besides, MEK5 downregulation also restrained the tumorigenesis of U2OS cells, while this alteration was reversed after LM22B-10 implement (Fig. 4C–E). In addition, MEK5 downregulation remarkedly weakened the mitochondrial membrane potential of U2OS cells (P < 0.01), while LM22B-10 treatment inhibited the decrease in mitochondrial membrane potential caused by MEK5 downregulation (P < 0.01; Fig. 4F). Taken together, MEK5/ERK pathway promotes Nur77 expression, tumorigenesis and mitochondrial function in U2OS cells.
Fig. 4.
MEK5/ERK pathway promotes Nur77 expression, tumorigenesis and mitochondrial function in U2OS cells. A Transfection efficiency measured by immunofluorescence; scale bar = 50 μm. B Western blotting detected MEK5, p-ERK5/ERK5, and Nur77 protein levels in U2OS cells. C Cell migration capability of U2OS cells was detected by Transwell assay; scale bar = 50 μm. D Cell invasive capability of U2OS cells was detected by Transwell assay; scale bar = 50 μm. E Cell viability of U2OS cells was detected by CCK-8 assay. F Mitochondrial membrane potential levels of U2OS cells were detected by JC-1 assay. *P < 0.05, **P < 0.01
MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of U2OS cells
Based on above all, it is speculated that MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of OS cells. To verify this hypothesis, the Cytosporone B (Nur77 activator) was added. As shown in Fig. 5A, after Cytosporone B implement, the Nur77 protein expression was obviously elevated in sh-MEK5 group (P < 0.01). In addition, Cytosporone B implement significantly increased the tumorigenesis of U2OS cells in sh-MEK5 group (all P < 0.01; Fig. 5B–D). In addition, Cytosporone B implement inhibited the decrease in mitochondrial membrane potential caused by MEK5 downregulation (P < 0.01; Fig. 5E), and Cytosporone B implement also reversed the protein levels of mitophagy markers PINK1 and Parkin in sh-MEK5 group (P < 0.01; Fig. 5F). These findings confirmed that MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of U2OS cells.
Fig. 5.
MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of U2OS cells. A Western blotting detected Nur77 protein level in U2OS cells. B Cell viability of U2OS cells was detected by CCK-8 assay. C Cell migration capability of U2OS cells was detected by Transwell assay; scale bar = 50 μm. D Cell invasive capability of U2OS cells was detected by Transwell assay; scale bar = 50 μm. E Mitochondrial membrane potential levels of U2OS cells were detected by JC-1 assay. F Western blotting detected the protein levels of PINK1 and Parkin in U2OS cells. **P < 0.01
Discussion
OS is the commonest primary bone malignancy with a high tendency of local metastasis and invasion [32]. Numerous studies have revealed that MEK5/ERK5 pathway and mitophagy play significant regulatory roles in the development of cancer, containing OS [33, 34]. However, the potential molecular mechanisms in OS are still unclear. This study found that MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of OS cells.
Accumulating evidences have revealed that MEK5/ERK5 pathway has been recognized to play significant roles in several cancer markers and mediate the action of a range of oncogenes [13–15]. In this study, an obvious upregulation in the expression of MEK5, ERK5, and p-ERK5 in OS cell lines were observed when compared to those in normal control. Moreover, MEK5 downregulation also restrained the tumorigenesis of U2OS cells. These data illustrated that MEK5/ERK5 pathway is activated in OS, and MEK5/ERK pathway promotes tumorigenesis of U2OS cells, which were in line with the conclusions obtained in previous researches [35–37]. Besides, it’s revealed that mitophagy plays crucial roles in tumor occurrence and development [38], including OS. For example, Zheng et al. revealed that soy isoflavones mediated mitophagy via blocking the AKT/mTOR pathway to suppress the progression of OS [39]. Herein, MEK5 downregulation obviously weakened the mitochondrial membrane potential of U2OS cells, while LM22B-10 treatment inhibited the decrease in mitochondrial membrane potential caused by MEK5 downregulation. Craig et al. have illustrated that MEKK3–MEK5–ERK5 signaling facilitates mitochondrial degradation [16]. Thus, it’s suspected that MEK5/ERK pathway might promotes tumorigenesis of OS cells via regulating mitophagy.
Nur77, as known as NR4A1, plays meaningful role in many biological processes. It's reported that Nur77 is dysregulated in many cancers and is a vital therapeutic target in cancer [40, 41]. In this study, the Nur77 expression in the metastatic group was remarkedly higher than that in the primary group. Moreover, patients in high Nur77 expression group had lower survival rates than those in low expression group. These data suggested that Nur77 is an oncogene in OS. Li et al. have found that Nur77 facilitates tumorigenesis of gastric cancer cells [42], which is confirmed the results in this study. Besides, Nur77 closely related to mitophagy. Zhang et al. revealed that Nur77 through repressing Mfn2-mediated mitophagy to facilitate cerebral ischemia reperfusion injury [29]. Herein, Nur77 was significantly negatively correlated with a mitophagy-related pathway (GOBP MITOPHAGY) in OS. Therefore, Nur77 was significantly overexpressed and closely related to mitophagy and immune in OS. In addition, Nur77 was closely related to numerous immune cells, including activated dendritic cells, activated mast cells and M0 macrophages, and immune response gene sets chemokines and cytokines in OS. Dendritic cells are the professional antigen-presenting cells of the immune system, and it’s reported that nuclear receptor Nur77 deficiency alters the function of dendritic cell [43]. Macrophages have a fundamental role in the pathogenesis of OS [44]. Macrophages can be polarized from M0 macrophages into several phenotypes, including pro-inflammatory (M1) and anti-inflammatory (M2) types. Lith et al. revealed that nuclear receptor Nur77 regulates immunomechanics of macrophages [45]. In addition, Chen et al. have found that TIPE1 suppresses OS tumor growth by regulating macrophage infiltration [46]. As innate immune cells, mast cells can exert profound immunoregulatory effects on tumor progression by modulating angiogenesis [47]. In addition, Liu et al. illustrated that cytokines could be used as prognostic biomarkers in patients with OS [48]. These results might provide therapeutic targets for the OS immunotherapy. According to these conclusions, Nur77 could serve as a useful diagnostic tool and targets for developing anti-OS drugs. In addition, the US-FDA have approved some siRNA delivery systems for treating various diseases [2, 49, 50], whether nanoparticle loading siNur77 could be used for clinical therapy of OS need further explored. In addition, previous study have revealed that the expression of Nur77 is up-regulated when activated ERK5 signaling pathway [18]. This study also found that MEK5/ERK pathway promotes Nur77 expression. In addition, Cytosporone B implement significantly increased the tumorigenesis of U2OS cells in sh-MEK5 group, and inhibited the weaken in mitochondrial membrane potential caused by MEK5 downregulation, and reversed the protein levels of mitophagy markers PINK1 and Parkin in sh-MEK5 group. These data fully validate the proposed hypothesis, which suggested that MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of OS cells. Overall, this study provides a basis for future efforts to develop novel therapeutic strategies for OS.
However, although study confirmed that MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of OS cells, some limitations should be considered. First, the conclusions drew in this study were only via in vitro experiments, and in vivo experiments should also be conducted. In addition, some other detection indicators and methods of mitophagy should also be studied. Besides, other mitophagy markers, such as LC3 and Beclin1, should be further evaluated in this study. Finally, whether MEK5/ERK pathway and Nur77 could be used as the therapeutic target of OS should be deeply explored via clinical level.
Conclusion
In summary, MEK5/ERK5 pathway is activated in OS, and MEK5–ERK5 pathway mediates mitophagy by regulating Nur77 to promote tumorigenesis of OS cells. This study offers valuable understanding for the development of targeted therapeutic strategies for OS.
Acknowledgements
Not applicable.
Author contributions
Jianshu Wang: Conceptualization, Formal analysis, Methodology, Writing—original draft. Jinxu Xue: Data curation, Formal analysis, Methodology, Software. Baijing Ma: Data curation, Formal analysis, Software. Yanqi Zhu: Data curation, Software. Jing Li: Formal analysis, Methodology. Caiping Tian: Conceptualization, Formal analysis, Methodology, Writing—review & editing. All authors reviewed the manuscript.
Funding
Jianshu Wang, Caiping Tian, Baijing Ma, Jinxu Xue and Jing Li were supported by Gansu Province science and technology plan project (Grant Numbers 22JR5RA643), Jianshu Wang, Caiping Tian, Yanqi Zhu, Jinxu Xue, and Jing Li were supported by Gansu Province health industry outstanding young talents and backbone talents project (Grant Number GSWSQN2023-16).
Availability of data and materials
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
No approval of research ethics committees was required, because this article does not involve any animal or human experimentation.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shen S, Xu Y, Gong Z, Yao T, Qiao D, Huang Y, et al. Positive feedback regulation of circular RNA Hsa_circ_0000566 and HIF-1α promotes osteosarcoma progression and glycolysis metabolism. Aging Dis. 2023;14(2):529–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qin S, Wang Y, Ma C, Lv Q. Competitive endogenous network of circRNA, lncRNA, and miRNA in osteosarcoma chemoresistance. Eur J Med Res. 2023;28(1):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton BR, Schwarz R, Vatner R, Yeh B, Claude L, Indelicato DJ, et al. Osteosarcoma. Pediatric Blood Cancer. 2021;68(Suppl 2): e28352. [DOI] [PubMed] [Google Scholar]
- 4.Zhao X, Wu Q, Gong X, Liu J, Ma Y. Osteosarcoma: a review of current and future therapeutic approaches. Biomed Eng Online. 2021;20(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida A. Osteosarcoma: old and new challenges. Surg Pathol Clin. 2021;14(4):567–83. [DOI] [PubMed] [Google Scholar]
- 6.Xie L, Xu J, Li X, Zhou Z, Zhuang H, Sun X, et al. Complete remission of metastatic osteosarcoma using combined modality therapy: a retrospective analysis of unselected patients in China. BMC Cancer. 2021;21(1):337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S, Zhang H, Liu J, Shang G. Targeted therapy for osteosarcoma: a review. J Cancer Res Clin Oncol. 2023;149(9):6785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheng G, Gao Y, Yang Y, Wu H. Osteosarcoma and metastasis. Front Oncol. 2021;11:780264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li X, Ruan T, Wang S, Sun X, Liu C, Peng Y, et al. Mitochondria at the crossroads of cholestatic liver injury: targeting novel therapeutic avenues. J Clin Transl Hepatol. 2024;12(9):792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao R, Dong C, Liang Q, Gao J, Sun C, Gu Z, et al. Engineered mitochondrial transplantation as an anti-aging therapy. Aging Dis. 2024. 10.14336/AD.2024.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G, Zhao X, Bai Y, Liu J, Li W, Wu Y. Regulation of mitochondrial autophagy by lncRNA MALAT1 in sepsis-induced myocardial injury. Eur J Med Res. 2024;29(1):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He G, Pan X, Liu X, Zhu Y, Ma Y, Du C, et al. HIF-1α-mediated mitophagy determines ZnO nanoparticle-induced human osteosarcoma cell death both in vitro and in vivo. ACS Appl Mater Interfaces. 2020;12(43):48296–309. [DOI] [PubMed] [Google Scholar]
- 13.Paudel R, Fusi L, Schmidt M. The MEK5/ERK5 pathway in health and disease. Int J Mol Sci. 2021;22(14):7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simões AES, Rodrigues CMP, Borralho PM. The MEK5/ERK5 signalling pathway in cancer: a promising novel therapeutic target. Drug Discov Today. 2016;21(10):1654–63. [DOI] [PubMed] [Google Scholar]
- 15.Hoang VT, Yan TJ, Cavanaugh JE, Flaherty PT, Beckman BS, Burow ME. Oncogenic signaling of MEK5-ERK5. Cancer Lett. 2017;392:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig JE, Miller JN, Rayavarapu RR, Hong Z, Bulut GB, Zhuang W, et al. MEKK3-MEK5-ERK5 signaling promotes mitochondrial degradation. Cell Death Discov. 2020;6:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coon BG, Timalsina S, Astone M, Zhuang ZW, Fang J, Han J, et al. A mitochondrial contribution to anti-inflammatory shear stress signaling in vascular endothelial cells. J Cell Biol. 2022. 10.1083/jcb.202109144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Liang Y, Li Z, Xia N. Liraglutide promotes osteoblastic differentiation in MC3T3-E1 cells by ERK5 pathway. Int J Endocrinol. 2020;2020:8821077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer Cell. 2008;14(4):285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdóttir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27(12):1739–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clough E, Barrett T. The gene expression omnibus database. Methods Mol Biol. 2016;1418:93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7): e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao B, Liu L, Li A, Xiang C, Wang P, Li H, et al. Identification and Verification of immune-related gene prognostic signature based on ssGSEA for osteosarcoma. Front Oncol. 2020;10:607622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Therneau T, Lumley T. Survival: Survival Analysis. 2016.
- 25.Chen B, Khodadoust MS, Liu CL, Newman AM, Alizadeh AA. Profiling tumor infiltrating immune cells with CIBERSORT. Methods Mol Biol. 2018;1711:243–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang R, Gao W, Wang Z, Jian H, Peng L, Yu X, et al. Polyphyllin I induced ferroptosis to suppress the progression of hepatocellular carcinoma through activation of the mitochondrial dysfunction via Nrf2/HO-1/GPX4 axis. Phytomedicine. 2024;122:155135. [DOI] [PubMed] [Google Scholar]
- 28.Martínez LA, Álvarez AC. Quantification of the gene expression of nur77 in osteogenic osteosarcomas. Neurochem Res. 2010;92-B(1):81. [Google Scholar]
- 29.Zhang Z, Yu J. NR4A1 promotes cerebral ischemia reperfusion injury by repressing Mfn2-mediated mitophagy and inactivating the MAPK-ERK-CREB signaling pathway. Neurochem Res. 2018;43(10):1963–77. [DOI] [PubMed] [Google Scholar]
- 30.Pereira DM, Gomes SE, Borralho PM, Rodrigues CMP. MEK5/ERK5 activation regulates colon cancer stem-like cell properties. Cell Death Discov. 2019;5:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira DM, Rodrigues CMP. Targeted avenues for cancer treatment: the MEK5-ERK5 signaling pathway. Trends Mol Med. 2020;26(4):394–407. [DOI] [PubMed] [Google Scholar]
- 32.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–5. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Hu F. Mitophagy in tumor: foe or friend? Endokrynol Pol. 2023;74(5):511–9. [DOI] [PubMed] [Google Scholar]
- 34.Poole LP, Macleod KF. Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci CMLS. 2021;78(8):3817–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Fdez A, Re-Louhau MF, Rodríguez-Núñez P, Ludeña D, Matilla-Almazán S, Pandiella A, et al. Clinical, genetic and pharmacological data support targeting the MEK5/ERK5 module in lung cancer. NPJ Precis Oncol. 2021;5(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fukasawa K, Lyu J, Kubo T, Tanaka Y, Suzuki A, Horie T, et al. MEK5-ERK5 axis promotes self-renewal and tumorigenicity of glioma stem cells. Cancer Res Commun. 2023;3(1):148–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miranda M, Rozali E, Khanna KK, Al-Ejeh F. MEK5-ERK5 pathway associates with poor survival of breast cancer patients after systemic treatments. Oncoscience. 2015;2(2):99–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang B, Wei Y, Han T, Ji P, Miao H, Wu X, et al. LncRNA LBX2-AS1 promotes proliferation and migratory capacity of clear cell renal cell carcinoma through mitophagy. Eur J Med Res. 2024;29(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng Z, Zhao X, Yuan B, Jiang S, Yan R, Dong X, et al. Soy isoflavones induces mitophagy to inhibit the progression of osteosarcoma by blocking the AKT/mTOR signaling pathway. Mol Med. 2024;30(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.To SK, Zeng JZ, Wong AS. Nur77: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2012;16(6):573–85. [DOI] [PubMed] [Google Scholar]
- 41.Zhang W, Wu Z, Qi H, Chen L, Wang T, Mao X, et al. Celastrol upregulated ATG7 triggers autophagy via targeting Nur77 in colorectal cancer. Phytomedicine. 2022;104:154280. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Shi Y, Guo Y, Tian S. Nur77 promotes invasion and migration of gastric cancer cells through the NF-κB/IL-6 pathway. Nan fang yi ke da xue xue bao = J South Med Univ. 2022;42(9):1410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tel-Karthaus N, Kers-Rebel ED, Looman MW, Ichinose H, de Vries CJ, Ansems M. Nuclear receptor Nur77 deficiency alters dendritic cell function. Front Immunol. 2018;9:1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Zhang B, Zhang Q, Ma X, Feng H. Tumor-associated macrophages in osteosarcoma. J Zhejiang Univ Sci B. 2021;22(11):885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lith SC, Evers TMJ, Freire BM, van Tiel CM, Vos WG, Mashaghi A, et al. Nuclear receptor Nur77 regulates immunomechanics of macrophages. Eur J Cell Biol. 2024;103(2):151419. [DOI] [PubMed] [Google Scholar]
- 46.Chen P, Zhou J, Li J, Zhang Q, Zuo Q. TIPE1 suppresses osteosarcoma tumor growth by regulating macrophage infiltration. Clin Transl Oncol. 2019;21(3):334–41. [DOI] [PubMed] [Google Scholar]
- 47.Ribatti D, Tamma R, Vacca A. Mast cells and angiogenesis in human plasma cell malignancies. Int J Mol Sci. 2019;20(3):481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu G, Liu B, Liu B, Tang L, Liu Z, Dai H. Cytokines as prognostic biomarkers in osteosarcoma patients: a systematic review and meta-analysis. J Interf Cytokine Res. 2023;43(8):335–43. [DOI] [PubMed] [Google Scholar]
- 49.Chen Y, Huang Y, Li Q, Luo Z, Zhang Z, Huang H, et al. Targeting Xkr8 via nanoparticle-mediated in situ co-delivery of siRNA and chemotherapy drugs for cancer immunochemotherapy. Nat Nanotechnol. 2023;18(2):193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Z, Tang Y, Liu Y, Chen Z, Feng Y, Hu H, et al. Co-delivery of fucoxanthin and Twist siRNA using hydroxyethyl starch-cholesterol self-assembled polymer nanoparticles for triple-negative breast cancer synergistic therapy. J Adv Res. 2024. 10.1016/j.jare.2024.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.