Abstract
Chicken pepsinogen was incubated at pH2.5 with pepstatin. The zymogen activated itself by a sequential mechanism and an intact peptide derived from residues 1-26 in the protein was released in the first step. This peptide was found to inhibit the milk-clotting activities of pig and chicken pepsins and calf chymosin but to different extents.
Full text
PDF
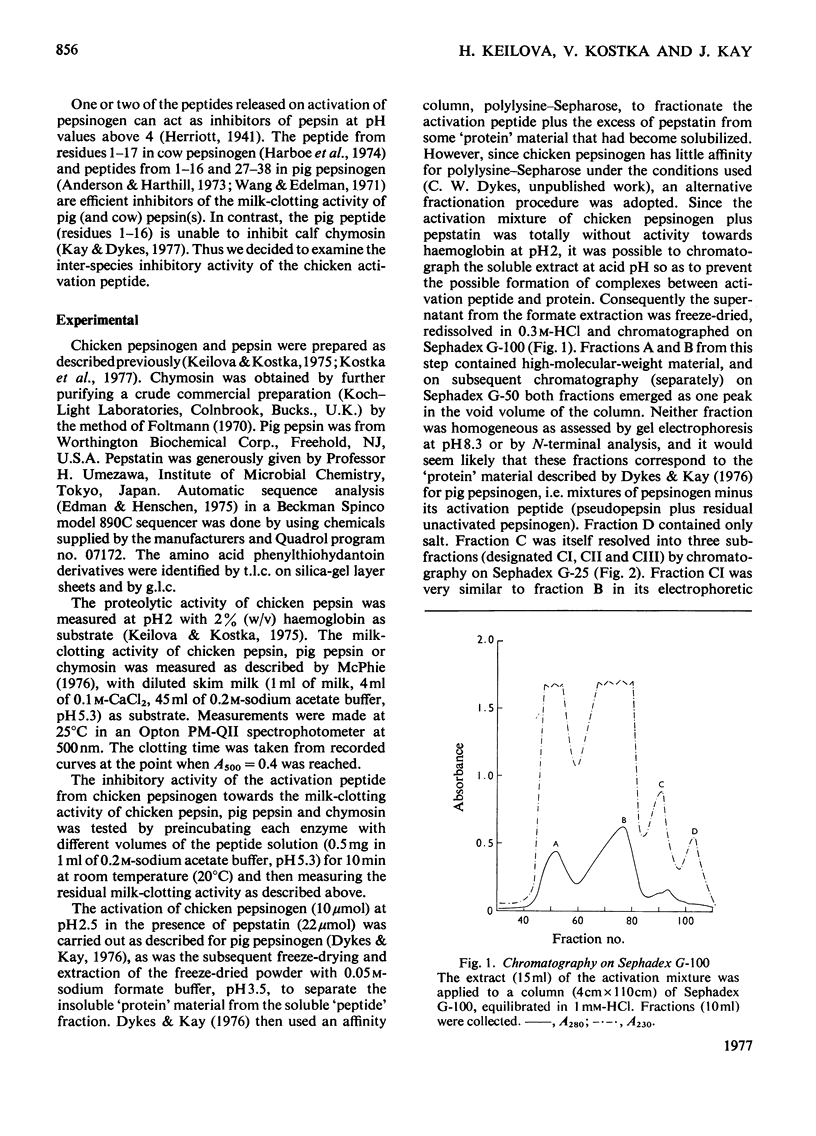
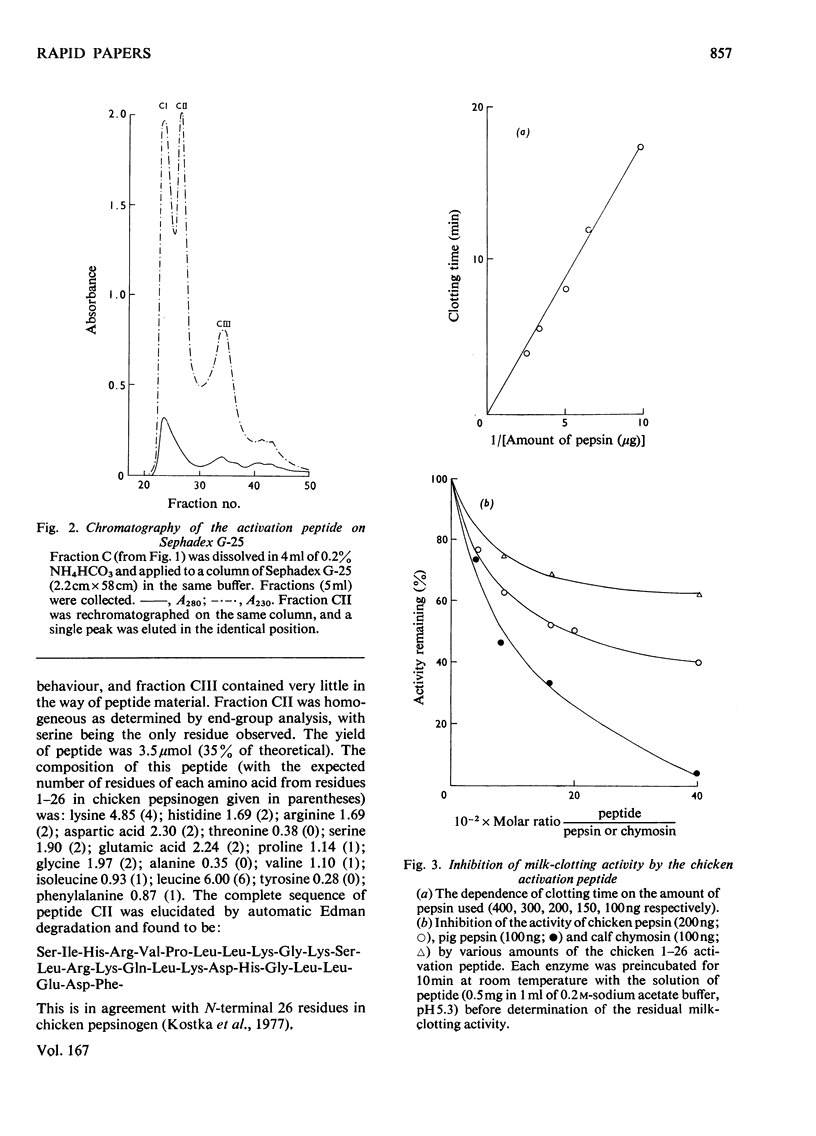

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W., Harthill J. E. Pepsin inhibitory activity amongst activation peptides of pepsinogen. Nature. 1973 Jun 15;243(5407):417–419. doi: 10.1038/243417b0. [DOI] [PubMed] [Google Scholar]
- Bohak Z. The kinetics of the conversion of chicken pepsinogen to chicken pepsin. Eur J Biochem. 1973 Feb 1;32(3):547–554. doi: 10.1111/j.1432-1033.1973.tb02640.x. [DOI] [PubMed] [Google Scholar]
- Christensen K. A., Pedersen V. B., Foltmann B. Identification of an enzymatically active intermediate in the activation of porcine pepsinogen. FEBS Lett. 1977 Apr 15;76(2):214–218. doi: 10.1016/0014-5793(77)80155-5. [DOI] [PubMed] [Google Scholar]
- Dykes C. W., Kay J. Conversion of pepsinogen into pepsin is not a one-step process. Biochem J. 1976 Jan 1;153(1):141–144. doi: 10.1042/bj1530141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruton J. S. The mechanism of the catalytic action of pepsin and related acid proteinases. Adv Enzymol Relat Areas Mol Biol. 1976;44:1–36. doi: 10.1002/9780470122891.ch1. [DOI] [PubMed] [Google Scholar]
- Harboe M., Andersen P. M., Foltmann B., Kay J., Kassell B. The activation of bovine pepsinogen. Sequence of the peptides released, identification of a pepsin inhibitor. J Biol Chem. 1974 Jul 25;249(14):4487–4494. [PubMed] [Google Scholar]
- Hsu I. N., Delbaere L. T., James M. N., Hofmann T. Penicillopepsin from Penicillium janthinellum crystal structure at 2.8 A and sequence homology with porcine pepsin. Nature. 1977 Mar 10;266(5598):140–145. doi: 10.1038/266140a0. [DOI] [PubMed] [Google Scholar]
- Kay J., Dykes C. W. Interaction of pepstatin with pig pepsinogen. Biochem J. 1976 Aug 1;157(2):499–502. doi: 10.1042/bj1570499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhie P. A turbidimetric milk-clotting assay for pepsin. Anal Biochem. 1976 May 21;73(1):258–261. doi: 10.1016/0003-2697(76)90166-4. [DOI] [PubMed] [Google Scholar]
- Subramanian E., Swan I. D., Liu M., Davies D. R., Jenkins J. A., Tickle I. J., Blundell T. L. Homology among acid proteases: comparison of crystal structures at 3A resolution of acid proteases from Rhizopus chinensis and Endothia parasitica. Proc Natl Acad Sci U S A. 1977 Feb;74(2):556–559. doi: 10.1073/pnas.74.2.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Edelman G. M. Fluorescent probes for conformational states of proteins. IV. The pepsinogen-pepsin conversion. J Biol Chem. 1971 Mar 10;246(5):1185–1191. [PubMed] [Google Scholar]


