Abstract
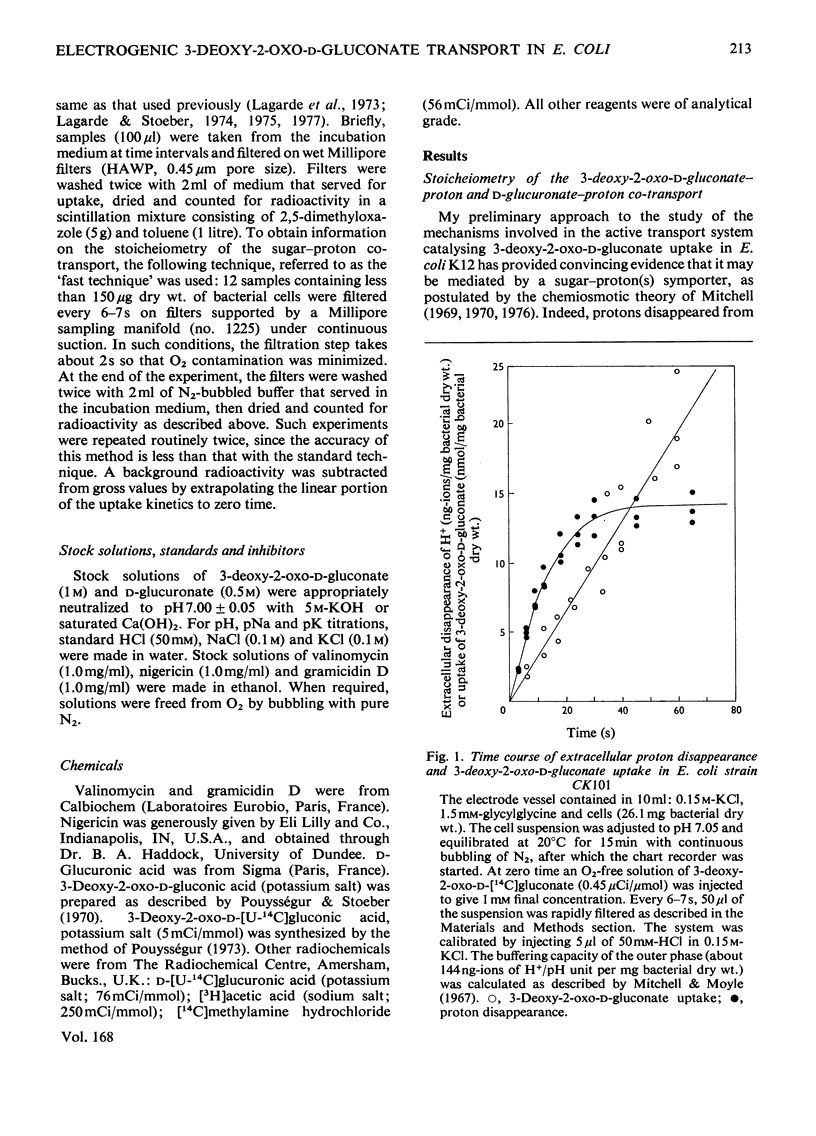
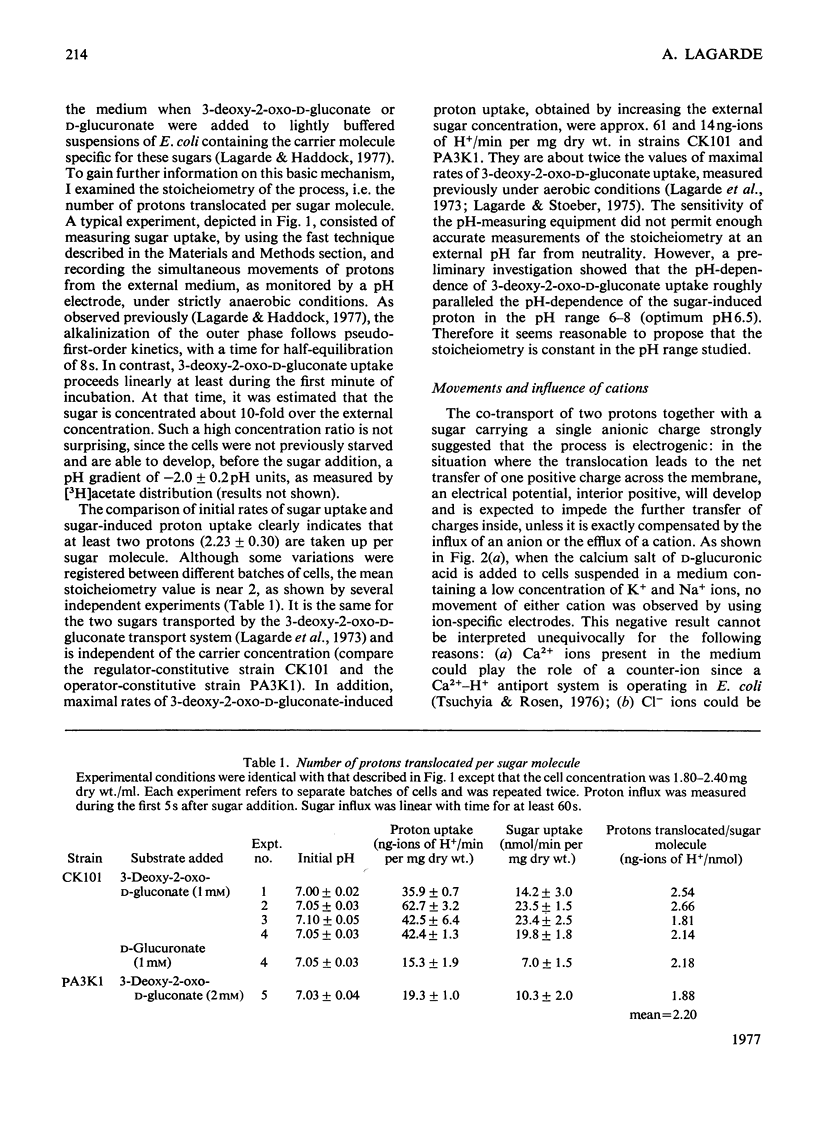
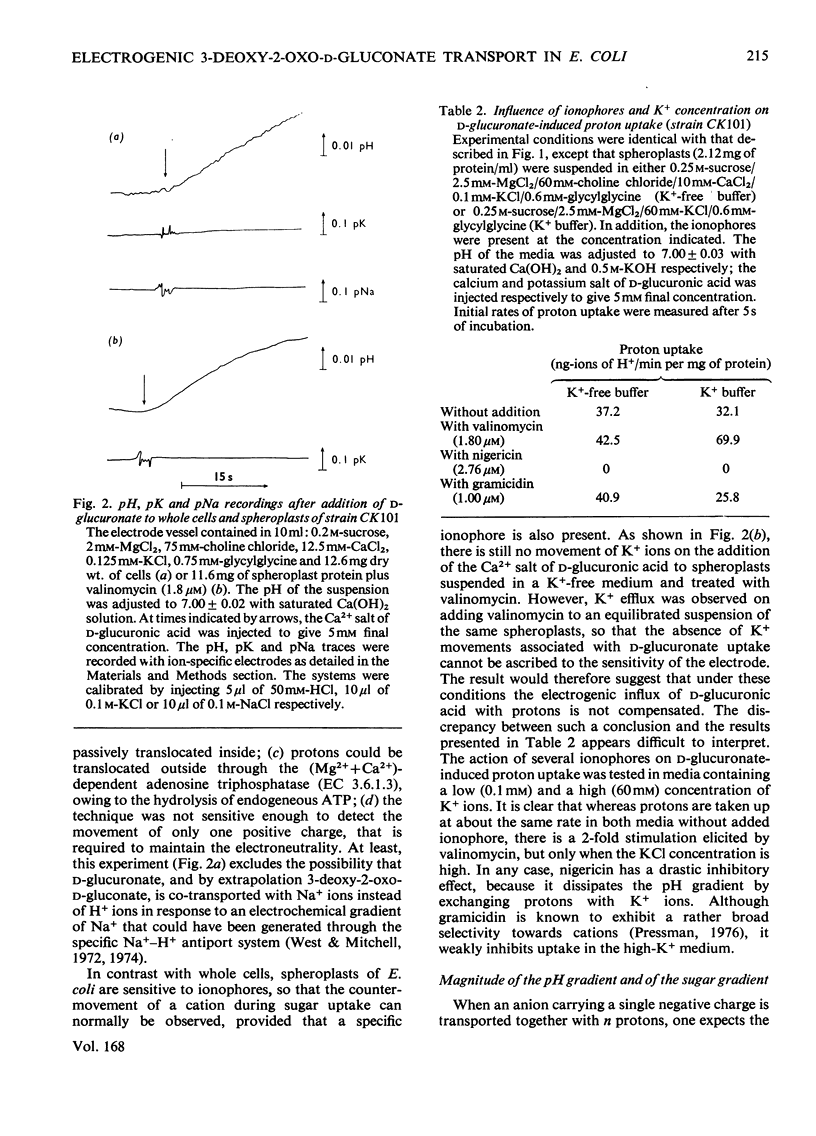
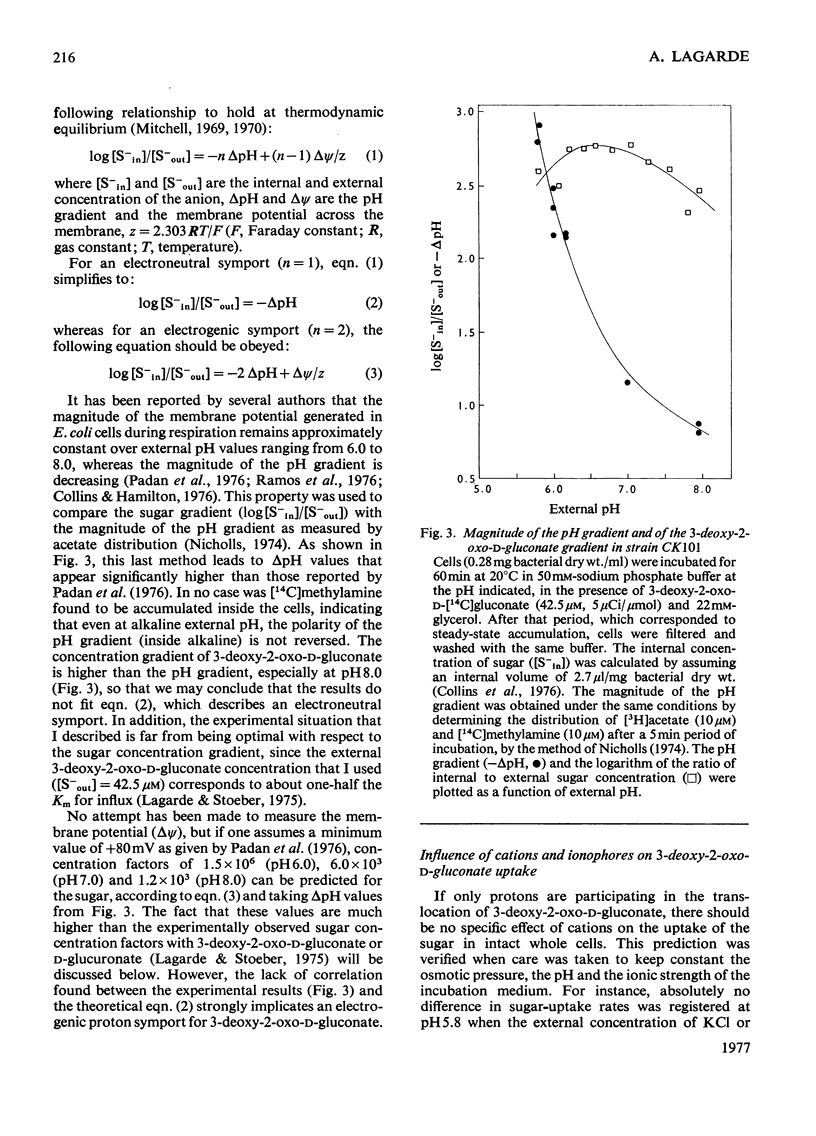
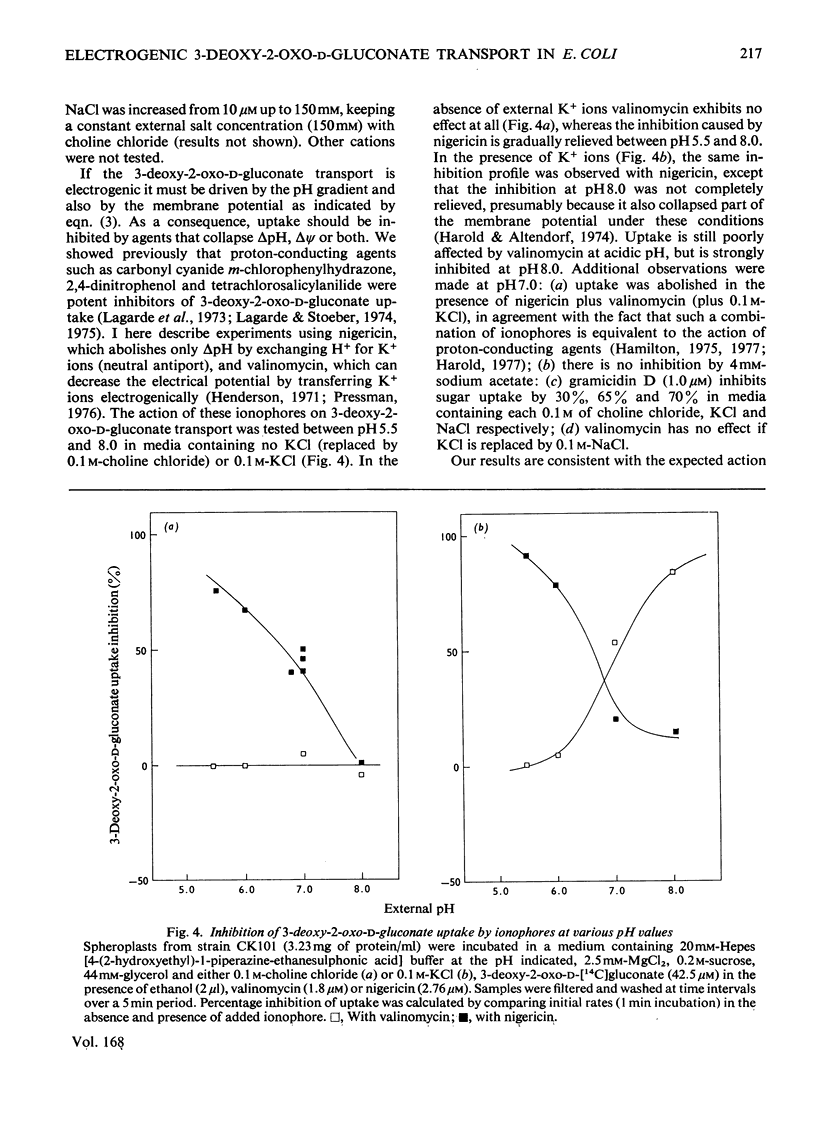
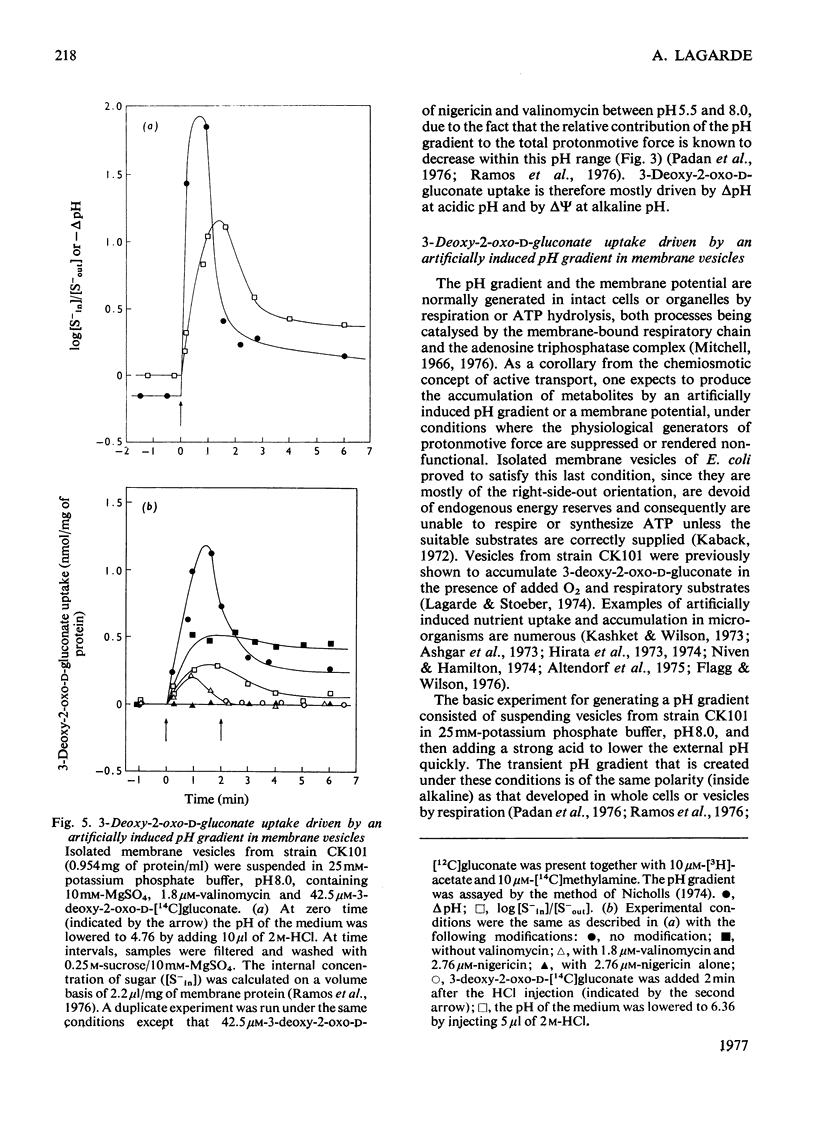
Evidence is presented indicating that the carrier-mediated uptake of 3-deoxy-2-oxo-D-gluconate and D-glucuronate in Escherichia coli K12 is driven by the deltapH and deltapsi components of the protonmotive force. 1. Approximately two protons enter the cells with each sugar molecule, independent of the sugar and the strain used. 2. In respiring cells, the magnitude of the pH gradient alone, as measured by distribution of [3H]acetate, appears to be insufficient to account for the chemical gradient of 3-deoxy-2-oxo-D-gluconate that is developed between pH 6.0 and 8.0. 3. If the external pH is varied between 5.5 and 8.0, 3-deoxy-2-oxo-D-gluconate uptake is gradually inhibited by valinomycin plus K+ ions, whereas the inhibition caused by nigericin is concomitantly relieved, thus reflecting the relative contribution of deltapH and deltapsi to the total protonmotive force at each external pH. 4. 3-Deoxy-2-oxo-D-gluconate can be transiently accumulated into isolated membrane vesicles in response to an artificially induced pH gradient. The process is stimulated when the membrane potential is collapsed by valinomycin in the presence of K+ ions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altendorf K., Hirata H., Harold F. M. Accumulation of lipid-soluble ions and of rubidium as indicators of the electrical potential in membrane vesicles of Escherichia coli. J Biol Chem. 1975 Feb 25;250(4):1405–1412. [PubMed] [Google Scholar]
- Asghar S. S., Levin E., Harold F. M. Accumulation of neutral amino acids by Streptococcus faecalis. Energy coupling by a proton-motive force. J Biol Chem. 1973 Aug 10;248(15):5225–5233. [PubMed] [Google Scholar]
- Booth I. R., Morris J. G. Proton-motive force in the obligately anaerobic bacterium Clostridium pasteurianum: a role in galactose and gluconate uptake. FEBS Lett. 1975 Nov 15;59(2):153–157. doi: 10.1016/0014-5793(75)80364-4. [DOI] [PubMed] [Google Scholar]
- Collins S. H., Hamilton W. A. Magnitude of the protonmotive force in respiring Staphylococcus aureus and Escherichia coli. J Bacteriol. 1976 Jun;126(3):1224–1231. doi: 10.1128/jb.126.3.1224-1231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. H., Jarvis A. W., Lindsay R. J., Hamilton W. A. Proton movements coupled to lactate and alanine transport in Escherichia coli: isolation of mutants with altered stoichiometry in alanine transport. J Bacteriol. 1976 Jun;126(3):1232–1244. doi: 10.1128/jb.126.3.1232-1244.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essenberg R. C., Kornberg H. L. Energy coupling in the uptake of hexose phosphates by Escherichia coli. J Biol Chem. 1975 Feb 10;250(3):939–945. [PubMed] [Google Scholar]
- Flagg J. L., Wilson T. H. Galactoside accumulation by Escherichia coli, driven by a pH gradient. J Bacteriol. 1976 Mar;125(3):1235–1239. doi: 10.1128/jb.125.3.1235-1236.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland P. B., Downie J. A., Haddock B. A. Proton translocation and the respiratory nitrate reductase of Escherichia coli. Biochem J. 1975 Dec;152(3):547–559. doi: 10.1042/bj1520547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geck P., Heinz E., Pfeiffer B. The degree and the efficiency of coupling between the influxes of Na + and -aminoisobutyrate in Ehrlich cells. Biochim Biophys Acta. 1972 Nov 2;288(2):486–491. doi: 10.1016/0005-2736(72)90272-6. [DOI] [PubMed] [Google Scholar]
- Grüneberg A., Komor E. Different proton-sugar stoichiometries for the uptake of glucose analogues by Chlorella vulgaris. Evidence for sugar-dependent proton uptake without concomitant sugar uptake by the proton-sugar symport system. Biochim Biophys Acta. 1976 Sep 21;448(1):133–142. doi: 10.1016/0005-2736(76)90082-1. [DOI] [PubMed] [Google Scholar]
- Gutowski S. J., Rosenberg H. Succinate uptake and related proton movements in Escherichia coli K12. Biochem J. 1975 Dec;152(3):647–654. doi: 10.1042/bj1520647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton G. M., Nicholls D. G. The calcium conductance of the inner membrane of rat liver mitochondria and the determination of the calcium electrochemical gradient. Biochem J. 1976 Jun 15;156(3):635–646. doi: 10.1042/bj1560635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz E., Geck P. The efficiency of energetic couping between Na+ flow and amino acid transport in Ehrlich cells-a revised assessment. Biochim Biophys Acta. 1974 Mar 29;339(3):426–431. doi: 10.1016/0005-2736(74)90170-9. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. Ion transport by energy-conserving biological membranes. Annu Rev Microbiol. 1971;25:393–428. doi: 10.1146/annurev.mi.25.100171.002141. [DOI] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Energy coupling in membrane vesicles of Escherichia coli. I. Accumulation of metabolites in response to an electrical potential. J Biol Chem. 1974 May 10;249(9):2939–2945. [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagarde A. E. A non-equilibrium thermodynamics analysis of active transport within the framework of the chemiosotic theory. Biochim Biophys Acta. 1976 Mar 5;426(2):198–217. doi: 10.1016/0005-2736(76)90332-1. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Haddock B. A. Proton uptake linked to the 3-deoxy-2-oxo-d-gluconate-transport system of Escherichia coli. Biochem J. 1977 Jan 15;162(1):183–187. doi: 10.1042/bj1620183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde A. E., Pouysségur J. M., Stoeber F. R. A transport system for 2-keto-3-deoxy-D-gluconate uptake in Escherichia coli K12. Biochemical and physiological studies in whole cells. Eur J Biochem. 1973 Jul 16;36(2):328–341. doi: 10.1111/j.1432-1033.1973.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. Escherichia coli K-12 structural kdgT mutants exhibiting thermosensitive 2-keto-3-deoxy-D-gluconate uptake. J Bacteriol. 1977 Feb;129(2):606–615. doi: 10.1128/jb.129.2.606-615.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. The energy-coupling controlled efflux of 2-keto-3-deoxy-D-gluconate in Escherichia coli K 12. Eur J Biochem. 1975 Jul 1;55(2):343–354. doi: 10.1111/j.1432-1033.1975.tb02168.x. [DOI] [PubMed] [Google Scholar]
- Lagarde A. E., Stoeber F. R. Transport of 2-keto-3-deoxy-D-gluconate in isolated membrane vesicles of Escherichia coli K12. Eur J Biochem. 1974 Mar 15;43(1):197–208. doi: 10.1111/j.1432-1033.1974.tb03400.x. [DOI] [PubMed] [Google Scholar]
- Lahav J., Essig A., Caplan S. R. The thermodynamic degree of coupling between metabolism and sodium transport in frog skin. Biochim Biophys Acta. 1976 Oct 5;448(2):389–392. doi: 10.1016/0005-2736(76)90251-0. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Roseman S. The bacterial phosphoenolpyruvate: sugar phosphotransferase system. Biochim Biophys Acta. 1976 Dec 14;457(3-4):213–257. doi: 10.1016/0304-4157(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J., Stoeber F. Synthèse enzymatique du 2-céto-3-désoxy-D-gluconate. Bull Soc Chim Biol (Paris) 1970;52(12):1419–1428. [PubMed] [Google Scholar]
- Pouysségur J., Lagarde A. Système de transport du 2-céto-3-désoxy-gluconate chez E. coli K 12: localisation d'un gène de structure et de son opérateur. Mol Gen Genet. 1973 Mar 1;121(2):163–180. doi: 10.1007/BF00277530. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin A., Kepes A. The mechanism of maintenance of electroneutrality during the transport of gluconate by E. coli. FEBS Lett. 1973 Oct 15;36(2):133–136. doi: 10.1016/0014-5793(73)80354-0. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. The driving force for proton(s) metabolites cotransport in bacterial cells. FEBS Lett. 1976 Jul 15;66(2):159–163. doi: 10.1016/0014-5793(76)80493-0. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. On the physical state of the intracellularly accumulates substrates of beta-galactoside-permease in Escherichia coli. Biochim Biophys Acta. 1958 Sep;29(3):579–587. doi: 10.1016/0006-3002(58)90015-5. [DOI] [PubMed] [Google Scholar]
- Seaston A., Carr G., Eddy A. A. The concentration of glycine by preparations of the yeast Saccharomyces Carlsbergensis depleted of adenosine triphosphate: Effects of proton gradients and uncoupling agents. Biochem J. 1976 Mar 15;154(3):669–676. doi: 10.1042/bj1540669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T., Rosen B. P. Calcium transport driven by a proton gradient and inverted membrane vesicles of Escherichia coli. J Biol Chem. 1976 Feb 25;251(4):962–967. [PubMed] [Google Scholar]
- West I. C., Mitchell P. Proton/sodium ion antiport in Escherichia coli. Biochem J. 1974 Oct;144(1):87–90. doi: 10.1042/bj1440087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I. C., Mitchell P. Stoicheiometry of lactose-H+ symport across the plasma membrane of Escherichia coli. Biochem J. 1973 Mar;132(3):587–592. doi: 10.1042/bj1320587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]



