Abstract
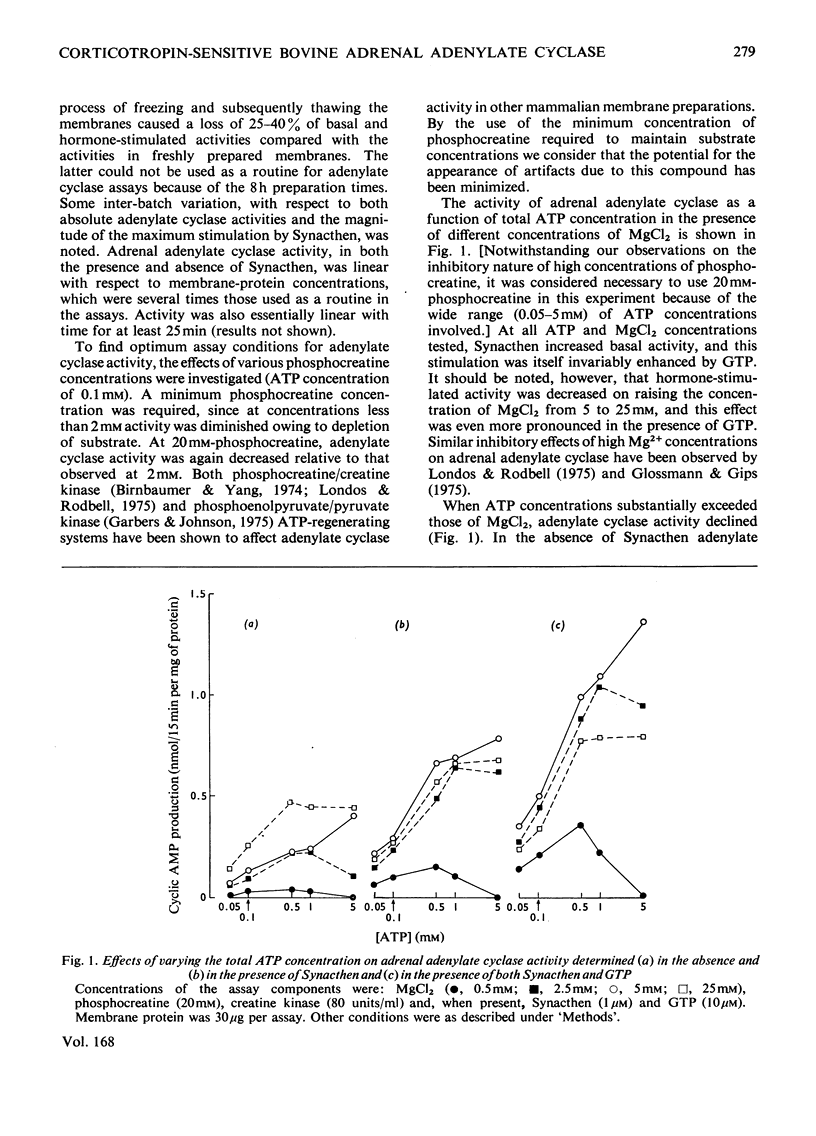
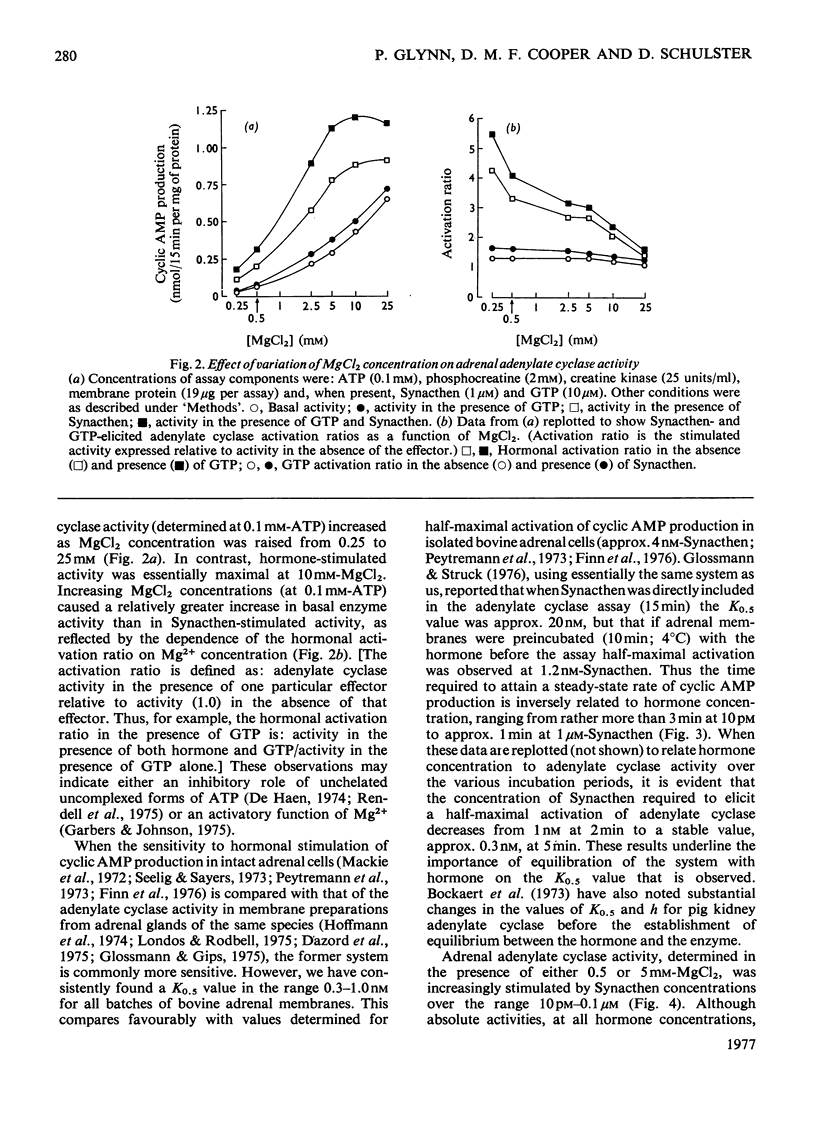
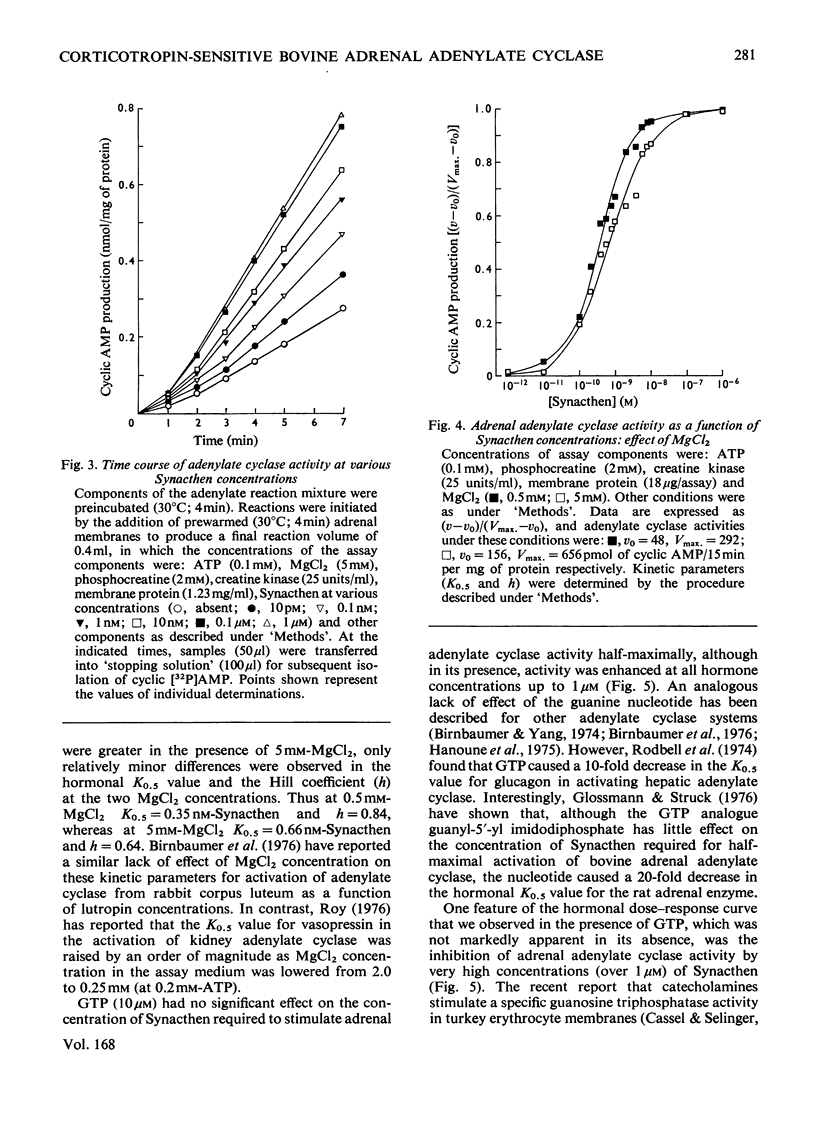
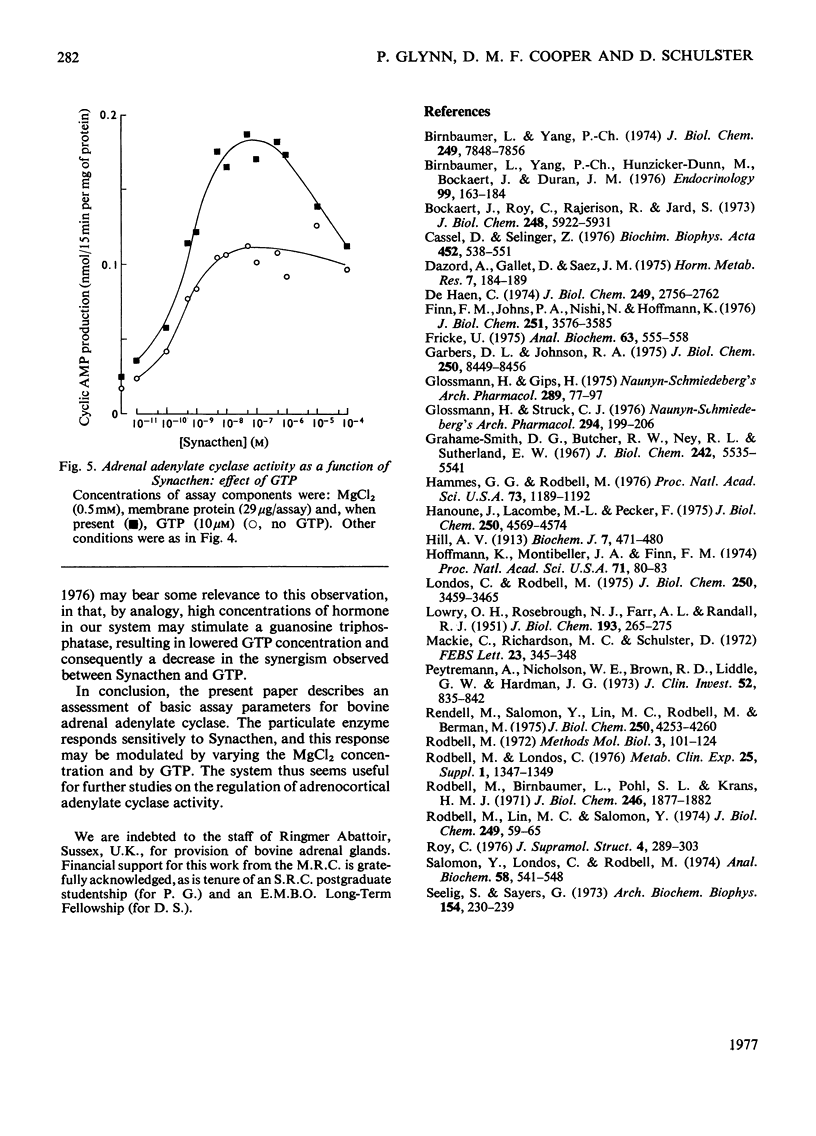
An assessment was made of some of the basic parameters responsible for the modulation of adenylate cyclase activity in a bovine adrenocortical plasma-membrane preparation. When determined at 0.1 mM-ATP, basal adenylate cyclase activity increased with increasing MgCl2 concentrations, whereas in the presence of corticotropin activity was essentially maximal at 10mM-MgCl2; high concentrations (25mM) of MgCl2 inhibited adenylate cyclase activity determined in the presence of both corticotropin and GTP. At all MgCl2 concentrations, corticotropin and GTP activated the enzyme in a synergistic fashion. The magnitude of the stimulation of basal activity produced by corticotropin was a function of Mg2+ concentration, whereas that produced by GTP appeared largely independent of Mg2+ concentration. Adenylate cyclase activity in the bovine adrenal membrane was half-maximally stimulated by corticotropin concentrations in the range 0.3--1.0 nM. The concentration of corticotropin evoking half-maximum response was not significantly affected by raising the free Mg2+ concentration from 0.4 to 4.9 mM, nor by the presence of GTP. In the presence of GTP, high concentrations (over 1 micrometer) of corticotropin inhibited adenylate cyclase activity, although no inhibition was apparent in the absence of guanine nucleotide.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Yang P. C., Hunzicker-Dunn M., Bockaert J., Duran J. M. Adenylyl cyclase activities in ovarian tissues. I. Homogenization and conditions of assay in graafian follicles and corpora lutea of rabbits, rats, and pigs: regulation by ATP, and some comparative properties. Endocrinology. 1976 Jul;99(1):163–184. doi: 10.1210/endo-99-1-163. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Yang P. C. Studies on receptor-mediated activation of adenylyl cyclases. I. Preparation and description of general properties of an adenylyl cyclase system in beef renal medullary membranes sensitive to neurohypophyseal hormones. J Biol Chem. 1974 Dec 25;249(24):7848–7856. [PubMed] [Google Scholar]
- Bockaert J., Roy C., Rajerison R., Jard S. Specific binding of (3H) lysine-vasopressin to pig kidney plasma membranes. Relationship of receptor occupancy to adenylate cyclase activation. J Biol Chem. 1973 Sep 10;248(17):5922–5931. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Finn F. M., Johns P. A., Nishi N., Hofmann K. Differential response to adrenocorticotropic hormone analogs of bovine adrenal plasma membranes and cells. J Biol Chem. 1976 Jun 25;251(12):3576–3585. [PubMed] [Google Scholar]
- Fricke U. Tritosol: a new scintillation cocktail based on Triton X-100. Anal Biochem. 1975 Feb;63(2):555–558. doi: 10.1016/0003-2697(75)90379-6. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Johnson R. A. Metal and metal-ATP interactions with brain and cardiac adenylate cyclases. J Biol Chem. 1975 Nov 10;250(21):8449–8456. [PubMed] [Google Scholar]
- Glossmann H., Gips H. Bovine adrenal cortex adenylate cyclase: properties of the particulate enzyme and effects of guanyl nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 1975;289(1):77–97. doi: 10.1007/BF00498031. [DOI] [PubMed] [Google Scholar]
- Glossmann H., Struck C. J. Adrenal cortex adenylate cyclase. In vitro acitivity of ACTH fragments and analogues. Naunyn Schmiedebergs Arch Pharmacol. 1976 Aug;294(2):199–206. doi: 10.1007/BF00507854. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Hammes G. G., Rodbell M. Simple model for hormone-activated adenylate cyclase systems. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1189–1192. doi: 10.1073/pnas.73.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J., Lacombe M. L., Pecker F. The epinephrine-sensitive adenylate cyclase of rat liver plasma membranes. Role of guanyl nucleotides. J Biol Chem. 1975 Jun 25;250(12):4569–4574. [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann K., Montibeller J. A., Finn F. M. ACTH antagonists. Proc Natl Acad Sci U S A. 1974 Jan;71(1):80–83. doi: 10.1073/pnas.71.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londos C., Rodbell M. Multiple inhibitory and activating effects of nucleotides and magnesium on adrenal adenylate cyclase. J Biol Chem. 1975 May 10;250(9):3459–3465. [PubMed] [Google Scholar]
- Mackie C., Richardson M. C., Schulster D. Kinetics and dose-response characteristics of adenosine 3',5'-monophosphate production by isolated rat adrenal cells stimulated with adrenocorticotrophic hormone. FEBS Lett. 1972 Jul 1;23(3):345–348. doi: 10.1016/0014-5793(72)80312-0. [DOI] [PubMed] [Google Scholar]
- Peytremann A., Nicholson W. E., Brown R. D., Liddle G. W., Hardman J. G. Comparative effects of angiotensin and ACTH on cyclic AMP and steroidogenesis in isolated bovine adrenal cells. J Clin Invest. 1973 Apr;52(4):835–842. doi: 10.1172/JCI107247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendell M., Salomon Y., Lin M. C., Rodbell M., Berman M. The hepatic adenylate cyclase system. III. A mathematical model for the steady state kinetics of catalysis and nucleotide regulation. J Biol Chem. 1975 Jun 10;250(11):4253–4260. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Roy C. Vasopressin-sensitive kidney adenylate cyclase: modulation of the hormonal response. J Supramol Struct. 1976;4(2):289–303. doi: 10.1002/jss.400040215. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Seelig S., Sayers G. Isolated adrenal cortex cells: ACTH agonists, partial agonists, antagonists; cyclic AMP and corticosterone production. Arch Biochem Biophys. 1973 Jan;154(1):230–239. doi: 10.1016/0003-9861(73)90053-2. [DOI] [PubMed] [Google Scholar]
- de Haën C. Adenylate cyclase. A new kinetic analysis of the effects of hormones and fluoride ion. J Biol Chem. 1974 May 10;249(9):2756–2762. [PubMed] [Google Scholar]


