Abstract
Background and Objectives
With upcoming clinical trials targeting preclinical stages of genetic frontotemporal dementia (FTD), early detection through cognitive screening is crucial. The Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) have potential as screening instruments for early-stage genetic FTD. However, no comparative evaluation has been performed. We aimed to compare MMSE and MoCA performance among presymptomatic, prodromal, and symptomatic pathogenic variant carriers to analyze which screening test has superior discriminative abilities.
Methods
We used cross-sectional and longitudinal data from 2 longitudinal genetic FTD cohort studies in the Netherlands and the United Kingdom, collected between 2021 and 2024. Participants were either presymptomatic, prodromal, or symptomatic pathogenic variant carriers or healthy controls (first-degree family members without pathogenic variants for FTD). Grouping was based on the global CDR-plus-NACC-FTLD score. Participants were assessed with both MoCA and MMSE. Statistical analyses compared total and subscores between groups and evaluated predictive and classification accuracy of both tests.
Results
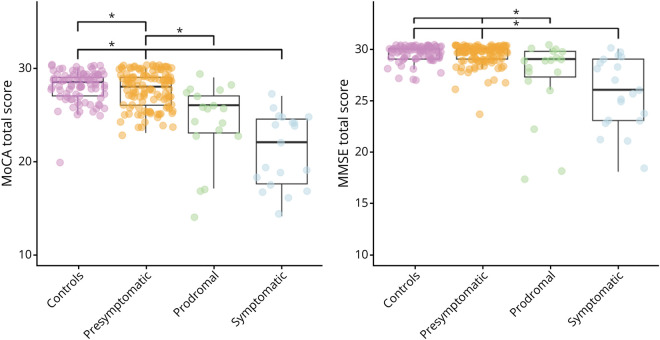
A total of 243 participants (mean age 49.9 ± 13.1 years, mean education 14.5 ± 3.0 years, 56% female), 157 of whom were pathogenic variant carriers (MAPT, GRN, C9orf72, TARDBP, and TBK1) and 86 controls, were included. Carriers were classified as presymptomatic (n = 119), prodromal (n = 18), or symptomatic (n = 20). Both MoCA [F(3,239) = 16.565, p < 0.001] and MMSE [F(3,239) = 13.529, p < 0.001] total scores differed significantly between groups, with controls (median MoCA 28.5, 95% CI 28.0–29.0; median MMSE 30, 95% CI 30.0–30.0) outperforming prodromal (median MoCA 26, 95% CI 23.0–27.0; median MMSE 29, 95% CI 27.5–29.5) and symptomatic (median MoCA 20.5, 95% CI 17.0–24.0; median MMSE 26, 95% CI 23.5–29.0) carriers. MoCA distinguished between presymptomatic carriers and controls (median MoCA 28, 95% CI 27.0–29.0), but MMSE did not. MoCA demonstrated superior discriminative ability compared with MMSE (MoCA area under the curve [AUC] = 0.87, 95% CI 0.81–0.94; MMSE AUC = 0.80, 95% CI 0.72–0.89).
Discussion
Its higher sensitivity and better discriminative power make MoCA a more valuable tool for cognitive screening in upcoming clinical trials targeting preclinical FTD. Future studies should aim for larger sample sizes from additional study centers.
Introduction
Frontotemporal dementia (FTD) is a clinically, pathologically, and genetically heterogeneous type of early-onset dementia, characterized by atrophy of primarily the frontal and/or temporal lobes.1 The clinical profile shows behavioral, language, and/or motor disturbances with cognitive deficits in executive function and social cognition, while memory and visuospatial abilities are relatively spared.2 In up to 40% of patients, an autosomal dominant pattern of inheritance is found. Pathogenic variants in the progranulin (GRN) and microtubule-associated protein tau (MAPT) gene and pathogenic G4C2 repeat expansions in chromosome 9 open reading frame 72 (C9orf72) are the most common causes.3 With promising avenues opening for clinical trials, research in the genetic FTD field has been increasingly moving toward the presymptomatic and early prodromal stages, in which the pathologic burden is still low.4 This highlights the importance of accurate cognitive screening in these early disease stages.
The Mini-Mental State Examination (MMSE)5 is one of the most widely used cognitive screening instruments for dementia. However, one of the main points of critique is the lack of tasks for executive function and the low complexity of the language tasks, which can be problematic when there are isolated problems in these 2 domains, for example, in early disease stages of FTD (often rendering MMSE performance normal).6 The newer Montreal Cognitive Assessment (MoCA)7 was developed to overcome the limitations of the MMSE by being able to detect more subtle cognitive deficits, as it comprises a more comprehensive assessment of the major cognitive domains, especially executive function and language, but also short-term memory and visuospatial processing.8 For any given MoCA score in dementia syndromes associated with a dysexecutive syndrome, including FTD, a trend toward a higher MMSE score was found.9 When comparing the psychometric properties and diagnostic accuracy of the MoCA and MMSE in patients with behavioral variant FTD (bvFTD), the MoCA demonstrated high diagnostic accuracy (i.e., area under the curve, AUC = 0.93), and using a cutoff of 17/30 proved to be superior to the MMSE in terms of classification accuracy.6 The MoCA demonstrated high diagnostic accuracy (i.e., area under the curve, AUC = 0.93), and using a cutoff of 17/30 proved to be superior to the MMSE in terms of classification accuracy. Despite its potential as a sensitive screening instrument for the early stages of FTD, no comparative evaluation of the MoCA and MMSE has been performed in presymptomatic or prodromal pathogenic variant carriers thus far.
The aim of this study was therefore to investigate differences in test performance on the MoCA and MMSE between presymptomatic, prodromal, and symptomatic pathogenic variant carriers, in comparison with cognitively healthy controls. In addition, we explored differences between pathogenic subtypes, predictive abilities and classification accuracy, and longitudinal effects for both cognitive screening instruments.
Methods
Participants
We included cross-sectional data of 125 participants from the FTD Risk Cohort (FTD-RisC) of the Erasmus MC University Medical Center (Erasmus MC, Rotterdam, the Netherlands) and 118 participants from the Dementia Research Center (DRC, University College London, London, United Kingdom). Both research sites are part of the Genetic FTD Initiative (GENFI) study, in which first-degree family members of patients with FTD due to a pathogenic variant are followed on a yearly basis.10 Data were gathered between September 2021 and March 2024. Pathogenic variant carriers were divided into 3 groups: presymptomatic carriers, prodromal carriers, and symptomatic carriers. Carriers (n = 119) were considered presymptomatic if they did not meet the clinical diagnostic criteria for bvFTD2 and had a global Clinical Dementia Rating scale plus National Alzheimer's Coordinating Center Frontotemporal Lobar Degeneration Behavior and Language domains (CDR-plus-NACC-FTLD11) score of 0. Eighteen carriers were considered prodromal because they met the following criteria: (1) progressive deterioration of behavior, language, and/or motor functioning; (2) functional decline, evidenced by multiple study visits with global CDR-plus-NACC-FTLD11 = 0.5 without reversing back to 0; and (3) cognitive decline, evidenced by ≥1.5 SD below age-specific, sex-specific, and education-specific means in ≥1 domain on neuropsychological assessment. Twenty carriers were considered symptomatic because they met diagnostic criteria for definite bvFTD.2 Diagnoses were made in multidisciplinary consensus meetings of the respective centers, using information from Clinical Assessment (see below). DNA genotyping assigned participants to the pathogenic variant (n = 157; C9orf72 (G4C2 expansions) n = 79, GRN n = 41, MAPT n = 34, TARDBP n = 2, TBK n = 1) or noncarrier group (controls; n = 86). Longitudinal data were available on 97 participants (C9orf72 n = 34, GRN n = 11, MAPT n = 21, TARDBP n = 2, controls n = 29).
Standard Protocol Approvals, Registrations, and Patient Consents
This observational study was conducted in accordance with the STROBE reporting guidelines. The investigators and participants were blinded for genetic status of at-risk participants, except for those who underwent predictive testing at their own request. All participants provided written informed consent. The Erasmus MC Medical Ethics Committee gave approval for the study (MEC 2009-409). At the DRC, ethical approval for the study was gained from the local research ethics committees (NRES Committee London - Queen Square, HRA NRES Center Manchester: 140377, London - Camden & Kings Cross Research Ethics Committee: 150805).
Clinical Assessment
All participants underwent a standardized clinical assessment, consisting of a structured interview with the participant and a knowledgeable informant (incorporating the CDR-plus-NACC-FTLD12), neurologic examination, neuropsychological assessment, and brain MRI. The neuropsychological assessment consisted of tests within the major cognitive domains (attention and mental processing speed, language, executive functioning, memory, visuospatial and visuoconstructive abilities, and social cognition). The MoCA6 and MMSE5 were administered as tests for global cognitive functioning. The Frontotemporal Dementia Rating Scale (FRS)13 was included to stage disease severity based on behavioral changes and functional decline. This caregiver questionnaire evaluates 7 aspects of a patient's personality and daily activities on a 3-point scale (never, sometimes, and all the time) reflecting the frequency of specific impairments. Scores were converted into percentages by dividing the total number of never responses by the total number of applicable questions, excluding items that were not applicable to the participant. A lower percentage indicates greater impairment of everyday abilities. The participants were grouped by the CDR-plus-NACC-FTLD; the MoCA and MMSE were not used in making this decision. The MoCA (version 8, 2018) covers 8 cognitive domains—visuospatial/executive, naming, memory, attention, language, abstract reasoning, delayed recall, and orientation—with a maximum score of 307. It adds 1 point for those who have fewer than 12 years of formal education.9 A score of ≥26 is considered normal.7 The MoCA can be administered in approximately 10 minutes.7 The MMSE covers 6 cognitive domains—orientation, registration, attention and calculation, recall, language, and visuospatial abilities—with a maximum score of 30. A score of ≥24 is considered normal. The MMSE can be administered in 5–10 minutes.14
Statistical Analysis
We conducted statistical analyses using SPSS Statistics (v28.0.1.0, IBM Corp., Armonk, NY) and created figures with R (v4.3.1, R Foundation for Statistical Computing, Vienna, Austria). Alpha was set at 0.05 across all comparisons (two-tailed), with Bonferroni corrections for multiple comparisons. Confidence intervals were provided for all parametric tests. We compared continuous demographic data between groups using one-way ANOVA for normally distributed data (with Bonferroni post hoc tests) or Kruskal-Wallis tests for non-normally distributed data (with Mann-Whitney U post hoc tests). Between-group differences in sex and pathogenic subtype (MAPT, GRN, C9orf72, TARDBP, and TBK) distribution were analyzed with Pearson χ2 tests. We used a general linear model with a log distribution to explore differences in MMSE and MoCA performance by study site (Rotterdam vs London) when correcting for sex, age, education, and CDR-plus-NACC-FTLD. Spearman correlations examined relationships between MoCA, MMSE, and FRS scores.
We compared performances on the MoCA and MMSE (total score and subtests) between clinical groups using nonparametric ANCOVA with age, sex, and education as covariates. We conducted an additional nonparametric ANCOVA incorporating FRS as a covariate to account for the effect of behavioral symptoms on MoCA/MMSE performance. Furthermore, we performed paired-sample t-tests to compare performances on MoCA and MMSE in every clinical group. Both ANCOVA and paired-sample t-test analyses were also performed in subsamples with ≤12 years (n = 84) and >12 years (n = 159) of education to explore the effects of low vs high education on MoCA/MMSE performance. We compared performances on the MoCA and MMSE between pathogenic subtypes (C9orf72, GRN, MAPT, TARDBP, and TBK were excluded because of a small sample size) using a nonparametric ANCOVA with age, sex, education, and CDR-plus-NACC-FTLD as covariates. To investigate whether the baseline MoCA or MMSE total scores had better predictive abilities, we built logistic regression models for each test, using group as the outcome variable, and calculated the Akaike information criterion (AIC) values for each model (AIC = 2*K – 2ln(L)). A model was considered significantly better when it was more than 2 AIC units lower than the other model. We determined sensitivity and specificity by area under the curve (AUC) by receiver-operating characteristics (ROC) analyses to investigate the classification abilities of the MoCA and MSE. An AUC >0.80 was considered to have excellent discrimination abilities.15 Cutoff levels for the total group and the subgroups with low and high education were given by the highest Youden Index.16 Finally, we conducted a generalized linear mixed model to evaluate decline (based on CDR-plus-NACC-FTLD global score) over time of the MoCA and MMSE, including age, education, and sex as covariates, with repeated measures on 2 time points.
Data Availability
Data not provided in the article may be shared (anonymized) at the request of a qualified investigator for purposes of replicating procedures and results.
Results
Demographics and Clinical Data
Demographic and clinical data are listed in Table 1. There were no differences between presymptomatic, prodromal, and symptomatic carriers in sex distribution [χ2(3) = 4.671, p = 0.198, 95% CI 0.186–0.202]. Symptomatic carriers had fewer years of education than presymptomatic carriers and controls [H(3) = 11.153, p = 0.011]. In addition, symptomatic carriers were older than both presymptomatic carriers [F(3,239) = 16.236, 95% CI 10.52–21.95, p < 0.001] and controls [F(3,239) = 10.423, 95% CI 4.55–16.29, p < 0.001]. Prodromal carriers were also older than presymptomatic carriers [F(3,239) = 14.681, 95% CI 8.70–20.66, p < 0.001] and controls [F(3,239) = 8.686, 95% CI 2.74–15.00, p = 0.005]. There were no significant differences between symptomatic carriers and prodromal carriers in age [F(3,239) = −1.555, 95% CI −6.13 to 9.24, p = 0.690]. Regarding differences between the 2 sites, we found significantly higher scores in the DRC (London) group on the MMSE total scores after correcting for age, sex, education, and CDR-plus-NACC-FTLD [β = −0.025, 95% CI −0.43 to 0.008, p = 0.005]. MoCA total scores were also higher in the DRC (London) group, but it was not significant after correcting for multiple testing [β = −0.30, 95% CI −0.060 to 0.000, p = 0.047]. The FRS score was significantly different in symptomatic and prodromal carriers compared with presymptomatic carriers and controls [H(3) = 45.760, p < 0.001). Furthermore, the FRS score correlated significantly with both MoCA (ρ = 0.330, 95% CI 0.181–0.464, p < 0.001) and MMSE (ρ = 0.207, 95% CI 0.050–0.354, p = 0.008) scores.
Table 1.
Demographic and Clinical Data per Subgroup
| Symptomatic pathogenic variant carriers (n = 20) | Prodromal pathogenic variant carriers (n = 18) | Presymptomatic pathogenic variant carriers (n = 119) | Controls (n = 86) | Statistical differences (p < 0.05) | |
| CDR-plus-NACC-FTLD | ≥1 | 0.5 | 0 | N/A | N/A |
| Subdomain scores (median, range) | N/A | N/A | |||
| Memory | 1 (0–3) | 0 (0–1) | 0 (0–0.5) | ||
| Orientation | 0.5 (0–2) | 0 (0–1) | 0 (0–0.5) | ||
| Judgment and problem solving | 1 (0–2) | 0 (0–0.5) | 0 (0–0.5) | ||
| Community affairs | 1 (0–3) | 0 (0–2) | 0 (0–0.5) | ||
| Home and hobbies | 1 (0–2) | 0 (0–1) | 0 (0–0.5) | ||
| Personal care | 0.5 (0–3) | 0 (0–1) | 0 (0) | ||
| Behavior | 1 (0–2) | 0 (0–0.5) | 0 (0–0.5) | ||
| Language | 0.5 (0-3 | 0.5 (0–0.5) | 0 (0–0.5) | ||
| Age at study entry, y | 61.6 ± 2.4 | 60.1 ± 2.6 | 45.4 ± 1.1 | 51.2 ± 1.4 | Sym, pro > con > pre |
| Sex, male/female | 11/9 | 4/14 | 52/67 | 40/46 | n.s. |
| Gene |
MAPT = 9 GRN = 2 C9orf72 = 8 TARDBP = 1 |
MAPT = 6 GRN = 5 C9orf72 = 6 TARDBP = 1 |
MAPT = 19 GRN = 34 C9orf72 = 65 TBK = 1 |
N/A | n.s. |
| Clinical diagnosis | bvFTD = 20 | N/A | N/A | N/A | N/A |
| Years of education | 12.7 ± 0.6 | 13.5 ± 0.8 | 14.9 ± 0.3 | 14.7 ± 0.3 | Sym < pre, con |
| Low (<12 y), n = 84 | 10.7 ± 1.6 (n = 12) | 10.8 ± 1.7 (n = 9) | 11.3 ± 1.4 (n = 36) | 10.9 ± 1.7 (n = 27) | n.s. |
| High (>12 y), n = 159 | 15.6 ± 0.7 (n = 8) | 16.2 ± 1.5 (n = 9) | 16.5 ± 1.6 (n = 83) | 16.4 ± 1.5 (n = 59) | n.s. |
| FRS total score | 49.1 ± 7.8 | 82.2 ± 4.9 | 92.8 ± 1.6 | 96.5 ± 0.8 | Sym = pro < pre = con |
| MoCA at baseline | |||||
| MoCA total [0–30] | 20.5 ± 5.0 | 24.2 ± 4.3 | 27.8 ± 1.9 | 28.1 ± 1.7 | Sym, pro < pre < con |
| Visuospatial/executive [0–5] | 3.3 ± 1.4 | 3.7 ± 0.7 | 4.5 ± 0.7 | 4.6 ± 0.5 | Sym < pre, con; pro < con |
| Naming [0–3] | 2.6 ± 0.6 | 2.8 ± 0.7 | 3.0 ± 0.2 | 3.0 ± 0.2 | Sym < pre, pro, con |
| Attention [0–6] | 4.8 ± 1.7 | 5.1 ± 1.3 | 5.9 ± 0.5 | 5.9 ± 0.4 | Sym, pro < pre, con |
| Language [0–3] | 1.5 ± 1.2 | 2.1 ± 0.9 | 2.7 ± 0.7 | 2.8 ± 0.5 | Sym, pro < pre, con |
| Abstraction [0–2] | 1.1 ± 0.8 | 1.5 ± 0.7 | 1.9 ± 0.4 | 1.8 ± 0.4 | Sym < pro, pre, con |
| Recall [0–5] | 1.7 ± 1.8 | 2.9 ± 2.1 | 3.8 ± 1.2 | 4.1 ± 1.1 | Sym < pro, pre, con; pre < con |
| Orientation [0–6] | 5.5 ± 1.0 | 5.8 ± 0.4 | 6.0 ± 0.1 | 5.9 ± 0.3 | Sym < pre |
| MMSE at baseline | |||||
| MMSE total [0–30] | 25.7 ± 3.4 | 27.2 ± 4.0 | 29.4 ± 1.0 | 29.6 ± 0.7 | Sym, pro < pre, con |
| Orientation [0–10] | 8.6 ± 1.5 | 9.40 ± 1.2 | 9.9 ± 0.4 | 9.8 ± 0.5 | Sym < pre, pro, con |
| Registration [0–3] | 3.0 ± 0.0 | 2.8 ± 0.7 | 3.0 ± 0.0 | 3.0 ± 0.0 | n.s. |
| Attention [0–5] | 4.2 ± 1.6 | 4.1 ± 1.6 | 4.8 ± 0.5 | 4.9 ± 0.3 | Pro < pre, con |
| Recall [0–3] | 2.2 ± 1.2 | 2.4 ± 1.0 | 2.9 ± 0.5 | 3.0 ± 0.2 | Sym < pre, con; pro < con |
| Language [0–8] | 7.0 ± 1.4 | 7.6 ± 0.9 | 7.9 ± 0.3 | 8.0 ± 0.2 | Sym < pre, pro, con; pro < pre, con |
| Construction | 0.8 ± 0.4 | 0.9 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.2 | Sym < pre, con |
| Follow-up (1 y) | Symptomatic pathogenic variant carriers (n = 9) | Prodromal pathogenic variant carriers (n = 11) | Presymptomatic pathogenic variant carriers (n = 47) | Controls (n = 30) | Statistical differences (p < 0.05) |
| CDR®-plus-NACC-FTLD global score | ≥1 | 0.5 | 0 | N/A | |
| Sex, male/female | 4/5 | 4/7 | 21/26 | 17/13 | N/A |
| Gene |
MAPT = 5 GRN = 1 C9 = 2 TARDBP = 1 |
MAPT = 3 GRN = 2 C9 = 5 TARDBP = 1 |
MAPT = 13 GRN = 8 C9 = 26 |
N/A | N/A |
| Clinical diagnosis | bvFTD = 9 | N/A | N/A | N/A | N/A |
Abbreviations: bvFTD = behavioral-variant frontotemporal dementia; C9orf72 = chromosome 9 open reading frame 72; CDR-plus-NACC-FTLD = Clinical Dementia Rating Scale plus National Alzheimer's Coordinating Center Frontotemporal Lobar Degeneration Behavior and Language domains; con = control; GRN = progranulin; MAPT = microtubule-associated protein tau; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment; n.s. = not significant; N/A = not applicable; pre = presymptomatic; pro = prodromal; sym = symptomatic.
Values indicate mean ± SD.
Group Comparison of the MoCA and MMSE Total Scores
Baseline MoCA total scores ranged between 7 and 30; baseline MMSE total scores ranged between 17 and 30. There were significant differences between groups in both total MoCA [F(3,239) = 16.565, p < 0.001] and MMSE [F(3,239) = 13.529, p < 0.001] scores (Figure 1). The MoCA total score was higher in controls (median MoCA 28.5, 95% CI 28.0–29.0) than in presymptomatic (median MoCA 28, 95% CI 27.0–29.0), prodromal (median MoCA 26, 95% CI 23.0–27.0), and symptomatic (median MoCA 20.5, 95% CI 17.0–24.0) carriers (p < 0.001–0.020). Similar patterns were found for the MMSE (median MMSE controls 30, 95% CI 30.0–30.0; prodromal 29, 95% CI 27.5–29.5; symptomatic 26, 95% CI 23.5–29.0), although the performance of presymptomatic carriers was not significantly different than of controls (p = 0.148). In addition, the variability in performance is higher for the MoCA compared with the MMSE in all groups. For both tests, no significant differences between prodromal and symptomatic carriers were found (p > 0.05). After correcting for FRS score, the statistical difference between presymptomatic carriers and controls on the MoCA and between presymptomatic and prodromal carriers on the MMSE did not remain significant. In all clinical groups, significant differences were found between MoCA and MMSE performance, with all MoCA total scores being lower than that of the MMSE (all p ≤ 0.001) (Figure 2). Subanalyses in participants with low (≤12 years) vs high (>12 years) education resulted in comparable findings, except for the statistical difference between presymptomatic carriers and controls not remaining significant in participants with >12 years of education (eTable 1).
Figure 1. Differences in MoCA and MMSE Performance Between Clinical Groups.
MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
Figure 2. Comparison MoCA and MMSE Between Clinical Groups.

MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
Group Comparison of the MoCA and MMSE Subscores
For the MoCA (eFigure 1), the visuospatial/executive subscore was lower in symptomatic carriers compared with presymptomatic carriers and controls [F(3,239) = 5.510, p = 0.00–0.006]. Prodromal carriers had lower scores than controls (p = 0.015). The attention subscore demonstrated differences in symptomatic and prodromal carriers when compared with presymptomatic carriers and controls [F(3,239) = 10.739, p < 0.001]. After FRS correction, only the difference between presymptomatic and prodromal carriers remained significant [F(3,158) = 2.802, p = 0.011]. Language performance was similarly affected, with symptomatic and prodromal carriers scoring lower than presymptomatic carriers and controls [F(3,239) = 12.468, p = 0.000–0.002]. The naming subscore also revealed differences, being lower in symptomatic carriers compared with prodromal and presymptomatic carriers and controls [F(3,239) = 9.222, p = 0.000–0.002]. After FRS correction, the difference between symptomatic and prodromal carriers did not remain significant. The abstraction subscore [F(3,239) = 6.483, p < 0.001] was lower in symptomatic carriers when compared with prodromal (p = 0.031) and presymptomatic carriers and controls (p < 0.001). These effects were not significant after correction for FRS. For the recall subscore [F(3,239) = 7.057], symptomatic carriers scored lower than presymptomatic carriers (p = 0.001) and controls (p < 0.001). In addition, presymptomatic carriers also scored lower on recall compared with controls (p = 0.042), although this difference was not significant after correction for multiple testing. After FRS correction, only the difference between symptomatic carriers and controls remained significant [F(3,158) = 1.698, p = 0.034]. Finally, the orientation subscore was lower in symptomatic carriers compared with presymptomatic carriers [F(3,239) = 3.576, p = 0.004], but these effects did not remain significant after FRS correction.
For the MMSE (eFigure 2), the orientation subscore [F(3,239) = 8.743] was lower in symptomatic carriers than prodromal carriers (p = 0.012), presymptomatic carriers (p < 0.001), and controls (p < 0.001). After FRS correction, only the difference between symptomatic carriers and presymptomatic carriers (p = 0.016) and controls (p = 0.014) remained significant [F(3,158) = 2.617]. No significant differences were observed in the registration subscore (p > 0.05). The attention subscore [F(3,239) = 3.420, p = 0.018] was lower in prodromal carriers when compared with presymptomatic carriers (p = 0.013) and controls (p = 0.002). After correcting for FRS score, these effects did not remain significant. The recall subscore [F(3,239) = 8.154, p < 0.001] was lower in symptomatic carriers than presymptomatic carriers and controls (both p < 0.001). Furthermore, prodromal carriers also scored lower on the recall subscore compared with controls (p = 0.011); however, this effect did not remain significant after correcting for FRS [F(3,158) = 2.752, p = 0.054]. In terms of language performance, this subscore [F(3,239) = 13.632, p < 0.001] was lower in symptomatic carriers compared with prodromal (p = 0.007) and presymptomatic carriers and controls (both p < 0.001). In addition, prodromal carriers had lower scores than controls (p = 0.012). After correcting for FRS, only the difference between symptomatic carriers and controls remained significant [F(3,158) = 2.429, p = 0.021]. The construction subscore [F(3,239) = 3.297, p = 0.021] was lower in symptomatic carriers compared with presymptomatic carriers (p = 0.003) and controls (p = 0.008).
Differences Between Pathogenic Subtypes on MoCA and MMSE Performance
Only the MoCA was able to distinguish between the different pathogenic subtypes [F(2,151) = 4.392, p = 0.014] (Figure 3). After correcting for CDR-plus-NACC-FTLD, the total MoCA performance was lower in the C9orf72 group compared with the GRN group (p = 0.004). In addition, only on the MoCA recall subscore were significant differences found [F(2,151) = 5.234, p = 0.006], with MAPT and C9orf72 carriers performing lower than the GRN carriers.
Figure 3. Differences in MoCA and MMSE Performance Between Genetic Groups.
C9orf72 = chromosome 9 open reading frame 72; CDR = CDR-plus-NACC-FTLD; GRN = progranulin; MAPT = microtubule-associated protein tau; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
Predictive Abilities and Classification Accuracy of the MoCA and MMSE
The χ2 test for both the MoCA model (χ2 = 240.484, df = 15, p < 0.001) and MMSE model (χ2 = 121.936, df = 15, p < 0.001) showed a statistically significant fit. For the MoCA, the beta coefficient was exp(β) = 0.852 (95% CI 0.67–1.08) in presymptomatic carriers vs controls, exp(β) = 0.451 (95% CI 0.28–0.73) for prodromal carriers vs controls, and exp(β) = 0.386 (95% CI 0.23–0.64) for symptomatic carriers vs controls. The beta coefficient for the MMSE was exp(β) = 0.686 (95% CI 0.44–1.08) in presymptomatic carriers vs controls, exp(β) = 0.382 (95% CI 0.21–0.70) for prodromal carriers vs controls, and exp(β) = 0.369 (95% CI 0.20–0.69) for symptomatic carriers vs controls. In both models, age and FRS were significant covariates (p < 0.001). The AIC suggested the MoCA to be a superior model (AIC MoCA 252.484, MMSE 262.867).
For distinguishing controls vs presymptomatic carriers, both MoCA (AUC = 0.534, 95% CI 0.456–0.613) and MMSE (AUC = 0.523, 95% CI 0.444–0.603) showed limited discriminative abilities. For distinguishing controls vs prodromal carriers, MoCA (AUC = 0.841, 95% CI 0.743–0.938) demonstrated better discriminative ability compared with MMSE (AUC = 0.752, 95% CI 0.613–0.897). Presymptomatic carriers vs prodromal carriers showed a similar result, although AUC values were lower (MoCA AUC = 0.793, 95% CI 0.689–0.898; MMSE AUC = 0.728, 95% CI 0.591–0.865). For distinguishing presymptomatic carriers vs prodromal carriers and symptomatic carriers, MoCA (AUC = 0.874, 95% CI 0.812–0.937) outperformed MMSE (AUC = 0.803, 95% CI 0.715–0.891), both showing an excellent discriminative ability. The ROC curve for the MoCA fully included the curve for the MMSE, indicating that there is always a cutoff for the MoCA with higher sensitivity and specificity, for any cutoff chosen for the MMSE (Figure 4). The optimal cutoff for distinguishing presymptomatic carriers from controls is 28 for the MoCA and 29 for the MMSE. For distinguishing controls and presymptomatic carriers from prodromal carriers, the optimal MoCA cutoff is 27 and the optimal MMSE cutoff is 29. For participants with ≤12 years of education, the optimal cutoff was 26 for the MoCA and 28 for the MMSE in distinguishing controls and presymptomatic carriers, 25 and 28, respectively, for controls vs prodromal carriers, and 25 for both tests for presymptomatic carriers vs prodromal and symptomatic carriers. See Table 2 for the diagnostic classification accuracy based on the highest Youden Index and eTable 2 for AUC values and optimal cutoff scores for participants with ≤12 years of education and participants with >12 years of education.
Figure 4. ROC Curves for Both MMSE and MoCA per Group.
MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
Table 2.
Sensitivity and Specificity of the Cutoff Scores With the Highest Youden Index
| Cutoff | Sensitivity | Specificity | AUC | |
| Controls vs presymptomatic | ||||
| MoCA | <28 | 0.71 | 0.41 | 0.53 |
| MMSE | <29 | 0.92 | 0.14 | 0.52 |
| Controls vs prodromal | ||||
| MoCA | <27 | 0.83 | 0.67 | 0.84 |
| MMSE | <29 | 0.92 | 0.44 | 0.75 |
| Presymptomatic vs prodromal | ||||
| MoCA | <27 | 0.74 | 0.67 | 0.79 |
| MMSE | <29 | 0.86 | 0.44 | 0.73 |
| Presymptomatic vs prodromal and symptomatic | ||||
| MoCA | <25 | 0.94 | 0.61 | 0.87 |
| MMSE | <28 | 0.86 | 0.58 | 0.80 |
Abbreviations: AUC = area under the operating characteristic curve; MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
MoCA and MMSE Performance Over Time
The effect of time was found to be statistically significant, indicating that MoCA and MMSE performance changed significantly between the 2 time points (Figure 5). The MoCA total score and time were significant predictors of the outcome variable. The coefficient was [β = −0.413, 95% CI −0.51 to −0.32, p < 0.001] for the MoCA total score and [β = −0.389, 95% CI −0.74 to −0.04, p = 0.031] for time. The same accounts for the MMSE model with total score and time coefficients of [β = −0.550, 95% CI −0.75 to −0.35, p < 0.001] and [β = −0.350, 95% CI −0.67 to −0.03, p = 0.035], respectively.
Figure 5. Mean Performance per Clinical Group.
MMSE = Mini-Mental State Examination; MoCA = Montreal Cognitive Assessment.
Discussion
With promising avenues opening for clinical trials, research in the genetic FTD field has been moving toward identifying sensitive and time-effective cognitive screening instruments for the presymptomatic and early prodromal stages.4,10 This study compared MoCA and MMSE performance across presymptomatic, prodromal, and symptomatic carriers of pathogenic variants and healthy controls. MoCA scores were consistently lower than MMSE scores and differentiated better between presymptomatic carriers and controls. MoCA also showed greater sensitivity, especially in C9orf72 carriers compared with GRN carriers, and outperformed MMSE in detecting longitudinal early cognitive decline.
Our approach contrasts with other studies in MoCA/MMSE comparisons that mostly focused solely on patients in the symptomatic phase of FTD6,17-19 and not on the presymptomatic and early symptomatic (prodromal) stages. The main finding from our study was that the MoCA outperformed the MMSE—in all stages of disease—but was particularly useful in identifying global cognitive decline as early as the presymptomatic stage, whereas the MMSE could not. In the GENFI study, the MMSE was significantly lower in presymptomatic carriers than in controls; however this study found that only carriers with an estimation of minus 5 years of onset had a significant lower MMSE.10 Our results are in line with studies comparing the MoCA and MMSE in symptomatic FTD that consistently found MoCA to be a more sensitive instrument than the MMSE.18 The results of this study also coincide with studies comparing the MoCA and MMSE in other patient groups. Previous studies showed that MoCA is better able to detect mild cognitive impairment (MCI), a precursor of dementia, than MMSE, with AUCs of 0.856 and 0.883 for MoCA and AUCs of 0.736 and 0.780 for MMSE, respectively.20,21 In patients with Parkinson disease, MoCA also outperformed MMSE, with a higher percentage of correct diagnoses.22 Combined, ours and previous studies suggest that the MoCA is more sensitive than the MMSE for the detection of early-stage dementia. This is most likely because the MoCA is considered more difficult than the MMSE because items measuring orientation may be easier than items assessing visuospatial and executive function.23 Moreover, as the MoCA additionally comprises items for executive function and language, it is presumably better able to detect dementia syndromes specifically targeting these cognitive domains, as is the case for FTD.
All MoCA/MMSE subscores, except the registration subscore of the MMSE, were significantly lower in symptomatic carriers than in presymptomatic carriers and controls. Of interest, the visuospatial/executive, attention, recall, and language subscores of the MoCA and the attention, recall, and language subtests of the MMSE were significantly lower in prodromal carriers than in presymptomatic carriers and controls. The findings are partially in line with those of a previous study that found lower visuospatial/executive, attention, language, recall, and orientation subscores in MCI due to Parkinson disease compared with healthy controls.22 The affected subscores coincide with the domains known to be declining in the presymptomatic stage of genetic FTD.24 The recall subtest of the MoCA was the only subscore that discriminated presymptomatic carriers from controls; however, correction for multiple testing rendered this finding nonsignificant. One explanation for this trend is the higher number of words (i.e., higher cognitive load) that needs to be recalled in the MoCA as compared with the MMSE.7 Moreover, the presymptomatic group comprises carriers with MAPT, GRN, and C9orf72 variants that all can be accompanied by memory deficits—even as early as the presymptomatic stage.24-26 An interesting exploration in a future study would be to investigate how memory subscores of the MoCA are affected in these pathogenic variants in different disease stages, as previous studies have shown gene-specific memory profiles in presymptomatic and symptomatic genetic FTD.22,24,27
In addition, our findings demonstrate that the MoCA exhibits a stronger relationship with behavioral test scores than the MMSE, as measured by the FRS. This may be attributed to the MoCA's broad coverage of cognitive domains, such as executive functioning and attention, which are closely tied to behavioral regulation.28,29 When analyzing MoCA and MMSE scores across groups, we observed that the MoCA showed greater differentiation, reflecting its sensitivity to cognitive deficits closely aligned with behavioral symptomatology. In line with this hypothesis, after controlling for the FRS in our statistical analyses, the effect of the MoCA was reduced. This suggests that the MoCA captures aspects of cognitive impairment that overlap significantly with behavioral dysfunction, raising the need for caution when using these tools in predictive models or group comparisons.
The optimal cutoff scores for distinguishing groups are 28 (MoCA) and 29 (MMSE) for presymptomatic carriers vs controls and 27 (MoCA) and 29 (MMSE) for prodromal carriers vs presymptomatic carriers and controls. Although the MMSE has better sensitivity, it lacks specificity, making a cutoff of 29 impractical in clinical settings. The MoCA offers better specificity, but this specificity remains low for clinical use.30 Previous studies report a similar MoCA specificity in MCI and early AD.20-22 The cutoff scores of this study are consistent with a previous study that identified the same cutoff for the MoCA to detect FTD.31 In contrast, these cutoff scores are higher compared to those found in a previous study (17 and 26, respectively), potentially because of differences in education level in the two studies.6 Because our study included a substantial proportion of higher educated (>12 years of education, >70% of the sample) than lower educated (≤12 years of education) participants, it is possible that this has influenced our results. Indeed, when we explored these effects in a subgroup analysis in lower educated individuals, we observed lower MoCA and MMSE mean total scores and optimal cutoff scores. Because education level is one of the main indirect proxies of cognitive reserve,32 it is likely that higher educated participants have higher scores on the MoCA and MMSE, even in the presence of mild cognitive impairments. These findings underscore the importance of considering education level when interpreting cognitive tests because it may significantly influence its sensitivity and specificity in detecting cognitive impairments.
Furthermore, time is a significant predictor in our model, suggesting that with disease progression (i.e., higher CDR plus NACC-FTLD), both MoCA and MMSE scores decline. This trend is particularly evident in prodromal and symptomatic carriers on the MoCA and symptomatic carriers on the MMSE, showing significant decline in performance from baseline to follow-up. Our longitudinal findings are in line with a previous study that found steeper rates of decline in the MoCA compared with the MMSE in patients with early-stage bvFTD than patients with AD, potentially serving as a method for differential diagnosis.19
When comparing MoCA and MMSE performance across different pathogenic subtypes of FTD, our findings point in the direction of superior sensitivity of the MoCA in differentiating between these pathogenic subtypes compared with the MMSE. Specifically, overall MoCA performance was lower in the C9orf72 carriers compared with the GRN group. This aligns with previous studies describing widespread impairment covering multiple cognitive domains in this FTD pathogenic variant.24,25,27,33 Furthermore, significant differences between pathogenic subtypes were observed for the MoCA recall subscore, with both MAPT and C9orf72 carriers performing lower than GRN carriers. This coincides with the study by Poos et al.24,26 who found memory to be contributing the strongest to the gene-specific composite score in carriers with C9orf72 and MAPT pathogenic variants. This is likely related to the temporal lobe atrophy, associated with memory deficits, in C9orf72 and MAPT pathogenic variants.
A strength of this study is its large cohort of genetic FTD variant carriers from 2 major FTD research centers, matched with pathogenic-negative controls. This design enhances the robustness of our findings by controlling for potential genetic confounders. In addition, we classified carriers into presymptomatic, prodromal, and symptomatic stages based on CDR-plus NACC-FTLD global scores,12 offering a comprehensive view across the disease stages of FTD. Although genetic FTD is a rare neurodegenerative disease,3 one limitation lies in the small sample size, especially when stratified into different clinical groups, which might have affected the generalizability of our findings. As a result, all symptomatic carriers were pooled into one group, despite some being in the early stages (i.e., CDR-plus NACC-FTLD 1) and others being in later stages (i.e., CDR-plus NACC-FTLD >1) of disease. Moreover, we decided to pool the pathogenic subtypes (including all CDR-plus NACC-FTLD scores), which has increased statistical power, but could have influenced gene-specific analyses. Future studies should aim to recruit larger study groups, for instance, by collaborating with other study sites within the larger GENFI consortium and worldwide initiatives for genetic FTD (such as the Frontotemporal Preventive Initiative). Furthermore, given the MoCA and MMSE differences between the 2 cohorts, additional cohorts are needed to assess the generalizability of these findings across languages and cultures. Finally, investigating longitudinal data of multiple follow-ups would be recommended to delineate more accurate profiles of cognitive performance over time, aiding in early disease detection and monitoring progression in genetic FTD.
Overall, the MoCA demonstrates greater discriminative ability than the MMSE. Our findings indicate that incorporating the MoCA into routine screening assessments for individuals at risk of FTD, particularly when evaluating presymptomatic and prodromal stages, is recommendable. Future studies, including larger sample sizes and more follow-up measurements, are needed to provide better insight into the generalizability of the current findings.
Acknowledgment
The authors thank all the participants and their families for taking part in the FTD-RisC and GENFI study.
Glossary
- AIC
Akaike information criterion
- AUC
area under the curve
- C9orf72
chromosome 9 open reading frame 72
- FRS
Frontotemporal Dementia Rating Scale
- FTD
frontotemporal dementia
- FTD-RisC
FTD Risk Cohort
- GENFI
Genetic FTD Initiative
- GRN
progranulin
- MAPT
microtubule-associated protein tau
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- ROC
receiver-operating characteristics
Author Contributions
L. de Boer: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data. J.M. Poos: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. E. Van Den Berg: drafting/revision of the manuscript for content, including medical writing for content. J.F.H. De Houwer: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. T. Swartenbroekx: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. E.G.P. Dopper: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P. Boesjes: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. N. Tahboun: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design. A. Bouzigues: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. P.H. Foster: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. E. Ferry-Bolder: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. K. Adams-Carr: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. L.L. Russell: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. R.S. Convery: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data. J.D. Rohrer: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design. H. Seelaar: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design. L.C. Jiskoot: drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design; analysis or interpretation of data.
Study Funding
The FTD-RisC study is supported by Dioraphte Foundation grant 09-02-00, the Association for frontotemporal Dementias Research Grant 2009, the Netherlands organization for Scientific Research (NWO) grant HCMI 056-13-018, ZonMw Memorabel project numbers 733050103 and 733050813, the Bluefield project, JPND PreFrontAls consortium project number 733051042, and ZonMw Onderzoeksprogramma Dementie (project number 10510032120002). L. de Boer was personally supported for this project by Alzheimer Nederland (project WE.08-2023-05). The authors have no competing interests relevant to this article's content. The Dementia Research Centre is supported by Alzheimer's Research UK, the Alzheimer's Society, Brain Research UK, and the Wolfson Foundation. This work was supported by the NIHR UCL/H Biomedical Research Centre, the Leonard Wolfson Experimental Neurology Centre (LWENC) Clinical Research Facility, and the UK Dementia Research Institute, which receives its funding from UK DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society and Alzheimer's Research UK. J.D. Rohrer has received funding from a Miriam Marks Brain Research UK Senior Fellowship, an MRC Clinician Scientist Fellowship (MR/M008525/1), the NIHR Rare Disease Translational Research Collaboration (BRC149/NS/MH), the MRC UK GENFI grant (MR/M023664/1), the Bluefield Project, and the JPND GENFI-PROX grant (2019-02248).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Olney NT, Ong E, Goh SYM, et al. Clinical and volumetric changes with increasing functional impairment in familial frontotemporal lobar degeneration. Alzheimers Dement. 2020;16(1):49-59. doi: 10.1016/j.jalz.2019.08.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456-2477. doi: 10.1093/brain/awr179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rohrer JD, Warren JD. Phenotypic signatures of genetic frontotemporal dementia. Curr Opin Neurol. 2011;24(6):542-549. doi: 10.1097/WCO.0b013e32834cd442 [DOI] [PubMed] [Google Scholar]
- 4.Rohrer JD, Warren JD, Fox NC, Rossor MN. Presymptomatic studies in genetic frontotemporal dementia. Revue Neurologique. 2013;169(10):820-824. doi: 10.1016/j.neurol.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 6.Freitas S, Simões MR, Alves L, Duro D, Santana I. Montreal Cognitive Assessment (MoCA): validation study for frontotemporal dementia. J Geriatr Psychiatry Neurol. 2012;25(3):146-154. doi: 10.1177/0891988712455235 [DOI] [PubMed] [Google Scholar]
- 7.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. doi: 10.1111/J.1532-5415.2005.53221.X [DOI] [PubMed] [Google Scholar]
- 8.Ismail Z, Rajji TK, Shulman KI. Brief cognitive screening instruments: an update. Int J Geriatr Psychiatry. 2010;25(2):111-120. doi: 10.1002/GPS.2306 [DOI] [PubMed] [Google Scholar]
- 9.Bergeron D, Flynn K, Verret L, et al. Multicenter validation of an MMSE-MoCA conversion table. J Am Geriatr Soc. 2017;65(5):1067-1072. doi: 10.1111/JGS.14779 [DOI] [PubMed] [Google Scholar]
- 10.Rohrer JD, Nicholas JM, Cash DM, et al. Presymptomatic cognitive and neuroanatomical changes in genetic frontotemporal dementia in the Genetic Frontotemporal Dementia Initiative (GENFI) study: a cross-sectional analysis. Lancet Neurol. 2015;14(3):253-262. doi: 10.1016/S1474-4422(14)70324-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyagawa T, Brushaber D, Syrjanen J, et al. Utility of the global CDR® plus NACC FTLD rating and development of scoring rules: data from the ARTFL/LEFFTDS Consortium. Alzheimers Dement. 2020;16(1):106-117. doi: 10.1002/ALZ.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyagawa T, Brushaber D, Syrjanen J, et al. Use of the CDR® plus NACC FTLD in mild FTLD: data from the ARTFL/LEFFTDS consortium. Alzheimers Dement. 2020;16(1):79-90. doi: 10.1016/J.JALZ.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mioshi E, Hsieh S, Savage S, Hornberger M, Hodges JR. Clinical staging and disease progression in frontotemporal dementia. Neurology. 2010;74(20):1591-1597. doi: 10.1212/WNL.0B013E3181E04070 [DOI] [PubMed] [Google Scholar]
- 14.Chiu EC, Chien TH, Lee YC. Measurement equivalence between the original and estimated mini-mental state examination in people with dementia. Int J Environ Res Public Health. 2021;18(14):7616. doi: 10.3390/IJERPH18147616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosmer DWJr, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons; 2013. [Google Scholar]
- 16.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. doi: [DOI] [PubMed] [Google Scholar]
- 17.Chow TW, Hynan LS, Lipton AM. MMSE scores decline at a greater rate in frontotemporal degeneration than in AD. Dement Geriatr Cogn Disord. 2006;22(3):194-199. doi: 10.1159/000094870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman KKL, Coleman BL, MacKinley JD, Pasternak SH, Finger EC. Association between montreal cognitive assessment sub-item scores and corresponding cognitive test performance in patients with frontotemporal dementia and related disorders. Dement Geriatr Cogn Disord. 2017;43(3-4):170-179. doi: 10.1159/000457119 [DOI] [PubMed] [Google Scholar]
- 19.Kansal K, Sullivan AC, Onyike CU. P2-092: comparisons of trajectories of MMSE and MoCA scores in frontotemporal dementia and Alzheimer's disease. Alzheimers Dement. 2015;11(7S_Part_11):519. doi: 10.1016/J.JALZ.2015.06.629 [DOI] [Google Scholar]
- 20.Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kȩdziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. 2016;50(5):1039-1052. doi: 10.12740/PP/45368 [DOI] [PubMed] [Google Scholar]
- 21.Pinto TCC, Machado L, Bulgacov TM, et al. Is the Montreal cognitive assessment (MoCA) screening superior to the Mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int Psychogeriatr. 2019;31(4):491-504. doi: 10.1017/S1041610218001370 [DOI] [PubMed] [Google Scholar]
- 22.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73(21):1738-1745. doi: 10.1212/WNL.0B013E3181C34B47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasnacht JS, Wueest AS, Berres M, et al. Conversion between the montreal cognitive assessment and the mini-mental status examination. J Am Geriatr Soc. 2023;71(3):869-879. doi: 10.1111/JGS.18124 [DOI] [PubMed] [Google Scholar]
- 24.Poos JM, Moore KM, Nicholas J, et al. Cognitive composites for genetic frontotemporal dementia: GENFI-Cog. Alzheimers Res Ther. 2022;14(1):10. doi: 10.1186/S13195-022-00958-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiskoot LC, Panman JL, van Asseldonk L, et al. Longitudinal cognitive biomarkers predicting symptom onset in presymptomatic frontotemporal dementia. J Neurol. 2018;265(6):1381-1392. doi: 10.1007/S00415-018-8850-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poos JM, Russell LL, Peakman G, et al. Impairment of episodic memory in genetic frontotemporal dementia: a GENFI study. Alzheimers Dement (Amst). 2021;13(1):e12185. doi: 10.1002/DAD2.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poos JM, Jiskoot LC, Leijdesdorff SMJ, et al. Cognitive profiles discriminate between genetic variants of behavioral frontotemporal dementia. J Neurol. 2020;267(6):1603-1612. doi: 10.1007/S00415-020-09738-Y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16(3):174-180. doi: 10.1016/J.TICS.2012.01.006 [DOI] [PubMed] [Google Scholar]
- 29.Moheb N, Mendez MF, Kremen SA, Teng E. Executive dysfunction and behavioral symptoms are associated with deficits in instrumental activities of daily Living in frontotemporal dementia. Dement Geriatr Cogn Disord. 2017;43(1-2):89-99. doi: 10.1159/000455119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Fine JP. Assessing the dependence of sensitivity and specificity on prevalence in meta-analysis. Biostatistics. 2011;12(4):710-722. doi: 10.1093/BIOSTATISTICS/KXR008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman KKL, Coleman BL, Mackinley JD, Pasternak SH, Finger EC. Detection and differentiation of frontotemporal dementia and related disorders from Alzheimer disease using the montreal cognitive assessment. Alzheimer Dis Assoc Disord/ 2016;30(3):258-263. doi: 10.1097/WAD.0000000000000119 [DOI] [PubMed] [Google Scholar]
- 32.Le Carret N, Lafont S, Mayo W, Fabrigoule C, Mayo W, Fabrigoule C. The effect of education on cognitive performances and its Implication for the constitution of the cognitive reserve. Dev Neuropsychol. 2003;23(3):317-337. doi: 10.1207/S15326942DN2303_1 [DOI] [PubMed] [Google Scholar]
- 33.Poos JM, Macdougall A, Van Den Berg E, et al. Longitudinal cognitive changes in genetic frontotemporal dementia within the GENFI cohort. Neurology. 2022;99(3):281-295. doi: 10.1212/WNL.0000000000200384 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article may be shared (anonymized) at the request of a qualified investigator for purposes of replicating procedures and results.