Abstract
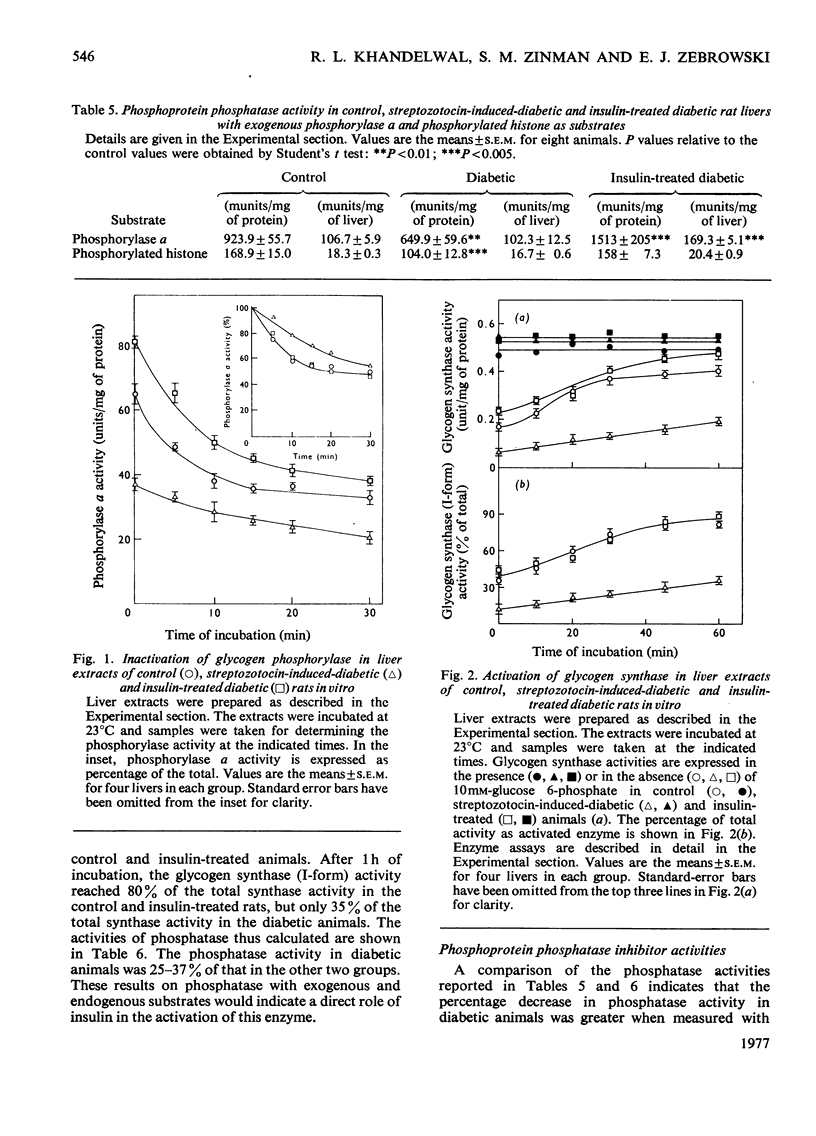
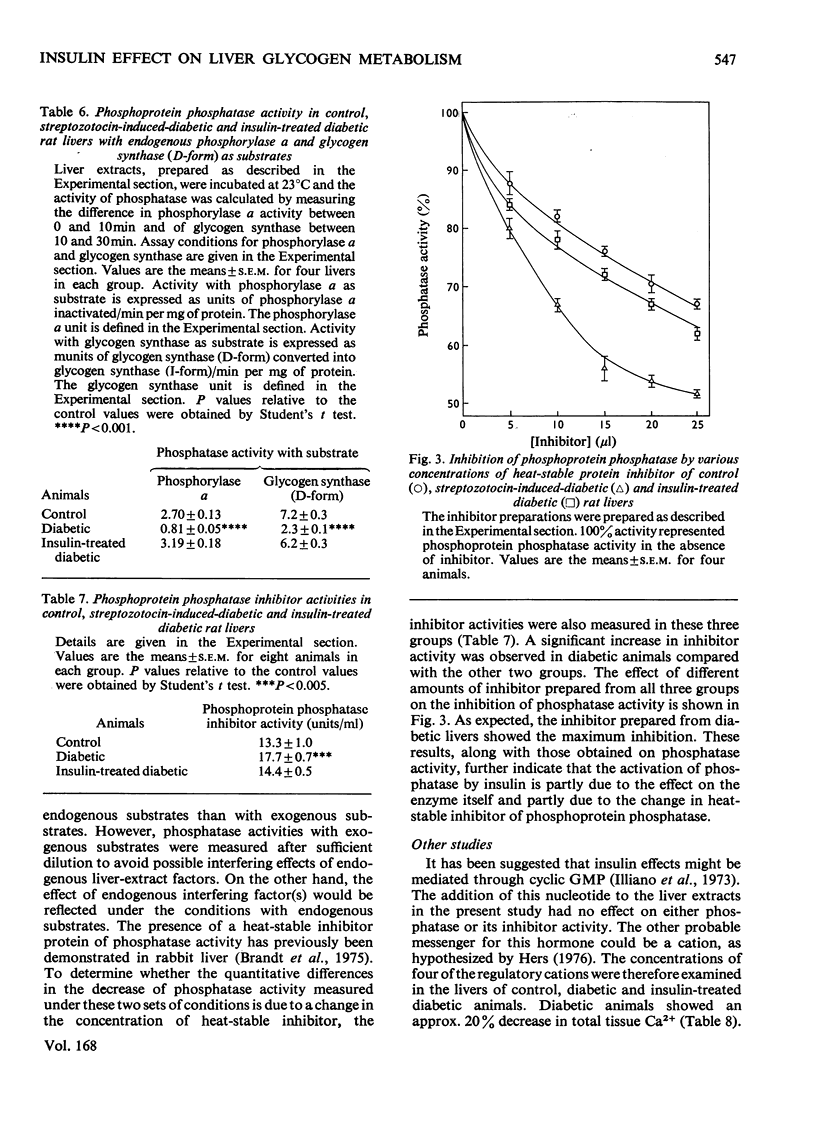
The effects of streptozotocin-induced diabetes and of insulin supplementation to diabetic rats on glycogen-metabolizing enzymes in liver were determined. The results were compared with those from control animals. The activities of glycogenolytic enzymes, i.e. phosphorylase (both a and b), phosphorylase kinase and protein kinase (in the presence or in the absence of cyclic AMP), were significantly decreased in the diabetic animals. The enzyme activities were restored to control values by insulin therapy. Glycogen synthase (I-form) activity, similarly decreased in the diabetic animals, was also restored to control values after the administration of insulin. The increase in glycogen synthase(I-form) activity after insulin treatment was associated with a concomitant increase in phosphoprotein phosphatase activity. The increase in phosphatase activity was due to (i) a change in the activity of the enzyme itself and (ii) a decrease in a heat stable protein inhibitor of the phosphatase activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. S., Goldberg N. D., Larner J. Insulin regulation of hepatic glycogen metabolism in the dog. Am J Physiol. 1971 Feb;220(2):499–506. doi: 10.1152/ajplegacy.1971.220.2.499. [DOI] [PubMed] [Google Scholar]
- Bishop J. S. Inability of insulin to activate liver glycogen transferase D phosphatase in the diabetic pancreatectomized dog. Biochim Biophys Acta. 1970 May 12;208(2):208–218. doi: 10.1016/0304-4165(70)90239-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. S., Larner J. Rapid activation-inactivation of liver uridine diphosphate glucose-glycogen transferase and phosphorylase by insulin and glucagon in vivo. J Biol Chem. 1967 Mar 25;242(6):1354–1356. [PubMed] [Google Scholar]
- Brandt H., Lee E. Y., Killilea S. D. A protein inhibitor of rabbit liver phosphorylase phosphatase. Biochem Biophys Res Commun. 1975 Apr 21;63(4):950–956. doi: 10.1016/0006-291x(75)90661-0. [DOI] [PubMed] [Google Scholar]
- Brostrom C. O., Hunkeler F. L., Krebs E. G. The regulation of skeletal muscle phosphorylase kinase by Ca2+. J Biol Chem. 1971 Apr 10;246(7):1961–1967. [PubMed] [Google Scholar]
- FISCHER E. H., KREBS E. G. The isolation and crystallization of rabbit skeletal muscle phosphorylase b. J Biol Chem. 1958 Mar;231(1):65–71. [PubMed] [Google Scholar]
- Glinsmann W. H., Mortimore G. E. Influence of glucagon and 3', 5'-AMP on insulin responsiveness of the perfused rat liver. Am J Physiol. 1968 Sep;215(3):553–559. doi: 10.1152/ajplegacy.1968.215.3.553. [DOI] [PubMed] [Google Scholar]
- Gold A. H., Segal H. L. Time-dependent increase in rat liver glycogen synthetase activity in vitro. Arch Biochem Biophys. 1967 May;120(2):359–364. doi: 10.1016/0003-9861(67)90251-2. [DOI] [PubMed] [Google Scholar]
- Gold A. H. The effect of diabetes and insulin on liver glycogen synthetase activation. J Biol Chem. 1970 Feb 25;245(4):903–905. [PubMed] [Google Scholar]
- Hayakawa T., Perkins J. P., Walsh D. A., Krebs E. G. Physiochemical properties of rabbit skeletal muscle phosphorylase kinase. Biochemistry. 1973 Feb;12(4):567–573. doi: 10.1021/bi00728a001. [DOI] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Howland B. E., Zebrowski E. J. Serum and pituitary gonadotropin levels in alloxan-diabetic rats. Horm Metab Res. 1974 Mar;6(2):121–124. doi: 10.1055/s-0028-1093874. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L., Glinsmann W. H., Robinson J. C. Regulation of glycogen synthetase activity by two kinases. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1163–1169. doi: 10.1016/s0006-291x(75)80351-2. [DOI] [PubMed] [Google Scholar]
- Illiano G., Tell G. P., Siegel M. E., Cuatrecasas P. Guanosine 3':5'-cyclic monophosphate and the action of insulin and acetylcholine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2443–2447. doi: 10.1073/pnas.70.8.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungas R. L. Role of cyclic-3',5'-amp in the response of adipose tissue to insulin. Proc Natl Acad Sci U S A. 1966 Aug;56(2):757–763. doi: 10.1073/pnas.56.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS E. G., KENT A. B., FISCHER E. H. The muscle phosphorylase b kinase reaction. J Biol Chem. 1958 Mar;231(1):73–83. [PubMed] [Google Scholar]
- KREBS E. G., LOVE D. S., BRATVOLD G. E., TRAYSER K. A., MEYER W. L., FISCHER E. H. PURIFICATION AND PROPERTIES OF RABBIT SKELETAL MUSCLE PHOSPHORYLASE B KINASE. Biochemistry. 1964 Aug;3:1022–1033. doi: 10.1021/bi00896a003. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Vandenheede J. R., Krebs E. G. Purification, properties, and substrate specificities of phosphoprotein phosphatase(s) from rabbit liver. J Biol Chem. 1976 Aug 25;251(16):4850–4858. [PubMed] [Google Scholar]
- Krebs E. G. Protein kinases. Curr Top Cell Regul. 1972;5:99–133. [PubMed] [Google Scholar]
- Kreutner W., Goldberg N. D. Dependence on insulin of the apparent hydrocortisone activation of hepatic glycogen synthetase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1515–1519. doi: 10.1073/pnas.58.4.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence J. C., Jr, Guinovart J. J., Larner J. Activation of rat adipocyte glycogen synthase by insulins. J Biol Chem. 1977 Jan 25;252(2):444–450. [PubMed] [Google Scholar]
- Mersmann H. J., Segal H. L. An on-off mechanism for liver glycogen synthetase activity. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1688–1695. doi: 10.1073/pnas.58.4.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. B., Jr, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem. 1973 May 25;248(10):3483–3488. [PubMed] [Google Scholar]
- Nuttali F. Q., Gannon M. C., Corbett V. A., Wheeler M. P. Insulin stimulation of heart glycogen synthase D phosphatase (protein phosphatase). J Biol Chem. 1976 Nov 10;251(21):6724–6729. [PubMed] [Google Scholar]
- Reimann E. M., Schlender K. K. Multiple forms of glycogen synthase kinase: isolation of forms which are independent of cyclic AMP. J Cyclic Nucleotide Res. 1976;2(1):39–46. [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. High affinity and low affinity binding of Ca++ by rat liver mitochondria. J Biol Chem. 1969 Feb 25;244(4):584–593. [PubMed] [Google Scholar]
- STEINER D. F., KING J. INDUCED SYNTHESIS OF HEPATIC URIDINE DIPHOSPHATE GLUCOSE-GLYCOGEN GLUCOSYLTRANSFERASE AFTER ADMINISTRATION OF INSULIN TO ALLOXAN-DIABETIC RATS. J Biol Chem. 1964 May;239:1292–1298. [PubMed] [Google Scholar]
- SUTHERLAND E. W., CORI C. F. Effect of hyperglycemic-glycogenolytic factor and epinephrine on liver phosphorylase. J Biol Chem. 1951 Feb;188(2):531–543. [PubMed] [Google Scholar]
- SUTHERLAND E. W. The effect of the hyperglycemic factor and epinephrine on enzyme systems of liver and muscle. Ann N Y Acad Sci. 1951 Dec;54(4):693–706. doi: 10.1111/j.1749-6632.1951.tb46623.x. [DOI] [PubMed] [Google Scholar]
- Sandham H. J., Kleinberg I. The effect of glucose concentration on the interrelation between glucose utilization, pH and carbohydrate storage in a salivary system. Arch Oral Biol. 1969 Jun;14(6):603–618. doi: 10.1016/0003-9969(69)90184-8. [DOI] [PubMed] [Google Scholar]
- Tan A. W., Nuttall F. Q. Characteristics of the dephosphorylated form of phosphorylase purified from rat liver and measurement of its activity in crude liver preparations. Biochim Biophys Acta. 1975 Nov 20;410(1):45–60. doi: 10.1016/0005-2744(75)90206-5. [DOI] [PubMed] [Google Scholar]
- Tan A. W., Nuttall F. Q. Regulation of synthase phosphatase and phosphorylase phosphatase in rat liver. Biochim Biophys Acta. 1976 Aug 12;445(1):118–130. doi: 10.1016/0005-2744(76)90165-0. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. Specificity of activation of glycogen synthase I from skeletal muscle and heart. Biochim Biophys Acta. 1973 Jan 12;293(1):84–93. doi: 10.1016/0005-2744(73)90378-1. [DOI] [PubMed] [Google Scholar]
- Torres H. N., Marechal L. R., Bernard E., Belocopitow E. Control of muscle glycogen phosphorylase activity by insulin. Biochim Biophys Acta. 1968 Feb 1;156(1):206–209. doi: 10.1016/0304-4165(68)90125-6. [DOI] [PubMed] [Google Scholar]
- de Wulf H., Hers H. G. The interconversion of liver glycogen synthetase a and b in vitro. Eur J Biochem. 1968 Dec 5;6(4):552–557. doi: 10.1111/j.1432-1033.1968.tb00480.x. [DOI] [PubMed] [Google Scholar]


