Abstract
The effect of aldehydes from lipid oxidation on the generation of heterocyclic amine 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) during pan-frying of golden pompano fillets was investigated. It was observed the aldehydes that contribute to thiobarbituric acid reactive substances (TBARS) value significantly promoted PhIP generation (p < 0.05). The defat treatment of fresh fish tissue significantly reduced the PhIP formation in pan-fried products (p < 0.05). During pan-frying, massive aldehydes were generated in a time-dependent manner with acrolein, propanal, hexanal, 4-hydroxy-nonenal (HNE), and 2,4-decadienal (DDE) as the abundant species. In the established chemical model, these aldehydes congruously promoted PhIP formation with the increased concentration, especially acrolein, HNE and DDE. Therefore, aldehydes could significantly enhance the PhIP generation under processing. However, except for propanal and hexanal, the promoting effect was slightly decreased with high levels of aldehydes addition due to the strong electrophilic properties and participation of reaction with the amino group of phenylacetaldehyde, creatinine, even PhIP.
Keywords: Golden pompano fillets, Lipid oxidation, Aldehydes, Heterocyclic aromatic amines
Highlights
-
•
-Aldehydes could contribute to PhIP formation by facilitating Strecker degradation of phenylalanine;
-
•
-Defat treatment could effectively reduce the PhIP generation during the pan-frying of fish pies;
-
•
-Aldehydes improved the PhIP generation in a concentration dependent manner in chemical model;
-
•
-Reactive aldehydes could interact with intermediate products of PhIP and affect the generation;
1. Introduction
Heterocyclic aromatic amines (HAAs) are generally produced during thermal processing of proteinaceous foods such as fish and meat, which are well-known for their hazardous effects including mutagenicity, neurotoxicity and carcinogenicity (Alaejos & Afonso, 2011; Geng et al., 2024; Zhao et al., 2024). Currently, more than 30 species of HAAs have been characterized in thermal-treated foods, which could be divided into Amino-imidazo-azaarenes and Amino-carbolines according to their structure (Wang et al., 2023). Evidences of epidemiological studies have indicated that the regular intake of thermally processed foods containing HAAs could increase the risk of diseases like Parkinson's disease or even human cancers including colon, liver, and mammary cancers (Bellamri et al., 2023; Xu et al., 2021; Zhao et al., 2021). Among the HAAs species, the 2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) has been classified as possible (class 2B) human carcinogens by the International Agency for Research on Cancer (Iarc, 1993).
Numerous of studies have reported the HAAs formation during heating of sugar, amino acids, and creatinine or by breaking down amino acids or proteins, though the complete formation mechanism remains to be fully elucidated (Hidalgo & Zamora, 2021; Wang et al., 2023; Zhang et al., 2024). PhIP, generally as the most concerned HAAs in thermally processed food, has achieved ever-increasing attention (Alaejos & Afonso, 2011; Chen, Jia, Zhu, Mao, & Zhang, 2020). The currently accepted mechanism indicated that phenylacetaldehyde formed by Strecker degradation of phenylalanine can react with creatinine to produce the aldol condensation product, which directly lead to the final PhIP formation through a series of reactions (Kang et al., 2022; Xu et al., 2023; Yang et al., 2021). Moreover, the carbonyl compounds generated by sugar decomposition have also been reported to facilitate this reaction (Gibis, 2016; Zamora & Hidalgo, 2015).
As for the complicate matrix, food lipids containing unsaturated fatty acids are susceptible to oxidative deterioration during thermal processing, which can result in the extensive generation of secondary oxidation products like aldehydes belong to reactive carbonyl species (RCS) (Ma, Liu, & Liu, 2019; Xu et al., 2022; Zhao et al., 2021). The RCS could help to enhance the Strecker degradation of phenylalanine into phenylacetaldehyde, thus contributing to the formation of PhIP (Zamora, Delgado, & Hidalgo, 2012). For example, acrolein, as a reactive α, β-unsaturated aldehyde generally formed during food thermal processing, was reported to contribute to the HAAs formation (Jiang, Jiang, Fan, Wang, & Zhao, 2022; Jing et al., 2022; Wang, Chu, et al., 2023). Moreover, the strong electrophilic properties of aldehydes also made them readily participate in the reaction with the amino group of creatinine, amino acid, even PhIP, which highlighted the complexity of food component interactions during thermal processing and the multi-dimensional mechanism on PhIP in products.
The golden pompano (Trachinotus blochii), an important marine fish preferably inhabits in tropical sea area, is beloved by consumers due to the desirable taste as well as abundant nutrients, such as high quality protein, polyunsaturated fatty acids (PUFAs) and mineral elements (Gao et al., 2024; Zhang et al., 2019). In our previous study, during the pan-frying process of golden pompano fillets, extensive PhIP formation, the dominant HAA species, have been observed (Zhao, Liu, Sun, et al., 2024). As for the fatty fish, PUFAs are susceptible to oxidative degradation and generation of oxidation products like aldehydes under the extreme condition (Gertz, Aladedunye, & Matthäus, 2014). However, the effect of aldehydes on the HAAs formation achieved little attention, especially for the dose-effect and structure-activity relationship, which remains unclear. Therefore, in the current study, the effect of lipid oxidation products aldehydes on the PhIP formation during pan-frying process of golden pompano fillets were investigated. Firstly, in terms of sample tissue in situ, the fillets after storage for different periods and with ever-climbing TBARS values were pan-fried to monitor the PhIP contents; In addition, the fresh defatted golden pompano steak were pan-fried with fresh fillets as control. Moreover, a chemical model simulating frying and containing glucose, phenylalanine and creatinine was established to observe the effect of aldehydes with different structures and a series of concentrations on the formation of PhIP. Simultaneously, the precursor like creatinine and intermediate product like phenylacetaldehyde were also determined to reveal the underlying mechanism.
2. Materials and methods
2.1. Materials
The fresh golden pompano, killed by trained personnel using decapitation, was purchased from a local aquatic market in Haikou, Hainan in March 2024, China and transferred to laboratory in the foam boxes with ice protection within 20 min. 2,4-dinitrophenylhydrazine (DNPH) and standards of DNPH derivatives for acrolein, propanal, butanal, crotonaldehyde, pentanal, hexanal, octanal, nonanal were obtained from Aladdin Reagent Co., Ltd. (Beijing, China). Aldehydes standards including acrolein, propanal, hexanal, DDE, HNE, and BF3-methanol (14 %, w/v) were obtained from Sigma-Aldrich (St. Louis, MO, USA). PhIP, phenylalanine, creatinine, and methanol, hexane, formic acid of high performance-liquid chromatography (HPLC) grade was obtained from Aladdin Reagent Co., Ltd. (Beijing, China). All other analytical grade reagents were from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Determination of TBARS values of golden pompano fillets
The golden pompano fillets with size of 6 × 6 × 1.5 cm stored in new polythene bags after refrigerated storage for 0, 1, 3, and 5 days were collected, storage at −80 °C and freeze-dried to obtain the powder for the simultaneous TBARS value determination. The TBARS values was determined by the method reported in our previous work (Gao et al., 2024), with the result expressed as μg malondialdehyde (MDA)/g dry golden pompano fillet powder.
2.3. Determination of fatty acid composition in fish fillets during storage
The lipid in fillets was extracted by the Bligh-Dyer method. The fatty acid composition of fish lipid was determined after methylation derivation to form fatty acid methylation esters (FAMEs). The FAMEs analysis was conducted by Agilent 7890B Gas Chromatography/7000B Mass Spectrometer (GC–MS) system (Palo Alto, CA, USA) equipped with a HP-5-MS capillary column (30 m × 0.25 mm, 0.25 μm) using the identical GC program and MS parameters as our previous work (Zhao et al., 2024). The levels of all fatty acids were measured using tridecanoic acid (C13:0) as an internal standard.
2.4. Defatting treatment of golden pompano fillets
To confirm the effect of lipid oxidation products such as aldehydes on the promotion of HAAs like PhIP, the intramuscular fat of fillet was removed and made into fish pies for pan-frying. The fresh golden pompano fillets were broken into paste by chopper mixer, then n-hexane was added for magnetic stirring to remove the intramuscular-fat by solvent extraction after adequate contact. Then the n-hexane was removed by centrifugation at 5000 rpm for 10 min. The defatted surimi was made into cylindrical fish pies with thickness of 1.5 cm by a specific mold. For the control group, fresh fillets were also broken into surimi and made into similar cylindrical fish pies for the pan-frying treatment.
2.5. Pan-frying of the refrigerated fillets and golden pompano fish pies
The refrigerated fillets with size of 6 × 6 × 1.5 cm and cylindrical fish pies made from a mold with diameter of 5 cm and thickness of 1.5 cm were pan-fried at 180 °C for 3, 5, 8 min and turn every 30 s to avoid burning with little palm oil to cover the pan bottom.
2.6. Determination of aldehydes content in pan-fried fillets by HPLC-MS/MS method
The aldehydes derived from lipid oxidation was derivatized with DNPH according to our established procedure (Zhao, Hu, et al., 2021) with minor modification. Briefly, total lipids were extracted by Bligh-Dyer method with mixture of chloroform and methanol (1:2, v/v). Then, 100 mg lipids were mixed with 2 mL DNPH solution (5 mg/mL in ethanol/formic acid = 9/1, v/v). After vortexing thoroughly, the mixture was incubated at 50 °C in dark for 2 h to obtain the dinitrophenylhydrazone, which was extracted by ethanol/water (1:1, v/v) for HPLC-MS/MS analysis.
The LC separation was conducted on a Shimadzu LC-40AVP system (Tokyo, Japan) equipped with Phenomenex Kinetex C18 column (150 mm × 2.1 mm, 1.7 μm, Torrance, CA, USA). The mobile phase A was H2O and mobile phase B was acetonitrile both containing 0.1 % (v/v) of formic acid. The gradient program was as follow: 0–2 min, 40–43 % B; 2–20 min, 43–90 % B; 20–22 min, 90 % B, 22–23 min, 90–40 % B, 23–23 min, 40 % B. The flow rate was 0.3 mL/min with injection volume of 5 μL. The MS detection was performed using a SCIEX Triple Quad 6500+ system by multiple reaction monitoring (MRM) mode with the transition ions established in our previous study (Zhao, Hu, et al., 2021). The instrument produced [M − H]− ions in negative mode with the following parameters: spray voltage of 4500 V, declustering potential of 90 V, collision cell exit potential of 15 V, entrance potential of 12 V, curtain gas (nitrogen) of 25 psi, nebulizer gas (GS1) of 45 psi, and turbo gas (GS2) of 40 psi.
2.7. Establishment of PhIP producing chemical model
A PhIP producing chemical model that containing 0.30 mmol phenylalanine and 0.30 mmol creatinine in 6 mL of diethylene glycol-ultrapure water mixture (5:1, v/v) into screw cap-sealed reaction vials was established according to our previous study (Zhao, Liu, Sun, et al., 2024). Different contents of acrolein (0, 0.03, 0.06, 0.12, and 0.24 mmol) were added, respectively, and heated at 170 °C for 30 min. After cooling with ice water, 1 mL of reaction mixture was mixed with 5 mL of ethyl acetate for PhIP extraction by vortexing under ultrasonication for 20 min, which was repeated for three times and the ethyl acetate phase was collected and rotary evaporated to remove the ethyl acetate. The residue was dissolved in 2 mL of methanol and filtered by 0.22 μm nylon 66 filter membranes (Jinteng, Tianjin, China) for HPLC-MS/MS analysis.
2.8. HPLC-MS analysis of PhIP, phenylacetaldehyde and creatinine
The PhIP in pan-fried fish fillets was extracted according to our previous study (Zhao, Liu, Sun, et al., 2024). HPLC-MS/MS analysis was conducted under the same conditions as described in the model system. For PhIP, phenylacetaldehyde and creatinine quantitation, the LC-MS/MS analysis was conducted. Derivatization of phenylacetaldehyde was conducted before injected into the HPLC-MS/MS system according to our previous study (Zhao, Liu, Sun, et al., 2024).
2.9. Statistical analysis
All experiments were performed at least in triplicate, and the data was analyzed using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). All results were expressed as mean ± standard deviation. Differences between different groups were analyzed by one-way analysis of variance (Duncan's multiple range tests). P < 0.05 was considered as statistically significant.
3. Results and discussion
3.1. Lipid oxidative deterioration of golden pompano fillets during refrigerated storage
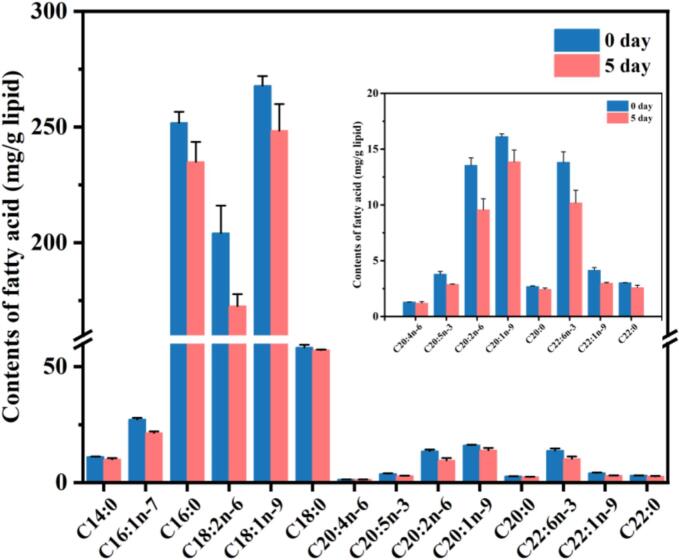
As shown in Fig. 1A, besides of the abundant protein, the golden pompano meat is rich in intramuscular lipids, especially long chain polyunsaturated fatty acids (LC-PUFAs), including C18:2 n-6, C20:2 n-6, C20:5 n-3, and C22:6 n-3 as well as monounsaturated fatty acids, such as C16:1 n-7, C18:1 n-9, and C20:1 n-9, which are susceptible to oxidative deterioration (Shahidi & Hossain, 2022; Wang, Liu, et al., 2023). Moreover, the continuous rising of fillets TBARS values from 0.62 to 1.09 mg MDA/kg after storage for 5 days were observed, which reflected the profound oxidation of fillets lipid (Fig. 2). From the fatty acid depletion perspective, the contents of C18:1 n-9, C16:0, C18:2 n-6, C18:0, C16:1 n-7, and C22:6 n-3 were decreased by 7.26 %, 6.70 %, 15.43 %, 1.89 %, 21.71 % and 10.18 %, respectively. Therefore, it was indicated that the oxidative degradation of UFAs caused large amount of aldehydes generation during the storage process.
Fig. 1.
Changes in fatty acid compositions (mg/g) of fish lipids during the refrigerated storage. Values were mean ± standard deviation, n = 3. 0 day: fresh golden pompano fillets; 5 day: golden pompano fillets after refrigerated storage for 5 day.
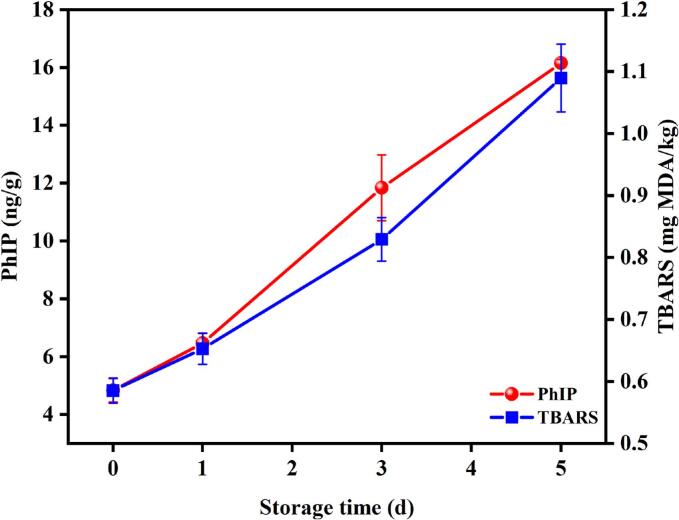
Fig. 2.
Changes in the TBARS values and PhIP contents during pan-frying of fish fillets after different periods of refrigerated storage. Values were mean ± standard deviation, n = 3.
3.2. Effects of lipid oxidation on the PhIP formation during pan-frying of refrigerated golden pompano fillets
The contents of PhIP during pan-frying of fillets after different periods of refrigerated storage were assessed and demonstrated in Fig. 2. As shown, for the fresh fillets, after pan-frying for 5 min, the detected PhIP content was 4.84 ng/g, with the extension of storage, the PhIP level of pan-fried fillets increased significantly. After storage for 5 days, the corresponding value increased to 16.15 ng/g, which was 3.33 folds higher than the fresh sample. Obviously, a positive correlation between the PhIP level and the TBARS value could be observed, which indicated that lipid oxidation products might be able to promote the generation of HAAs, like PhIP. In a previous study, Jing et al. (2022) reported that the tilapia fish lipid oxidation served as accelerator for PhIP formation during the roasting of fish patties. Thus, it could be assured that the lipid oxidation products could contribute to the formation of PhIP. In reverse thinking, the high detected level of HAAs in fried meat products could also reflect their freshness to certain extent, which can be helpful in food adulteration application.
3.3. Effect of defat treatment on the PhIP contents in the fillets during pan-frying process
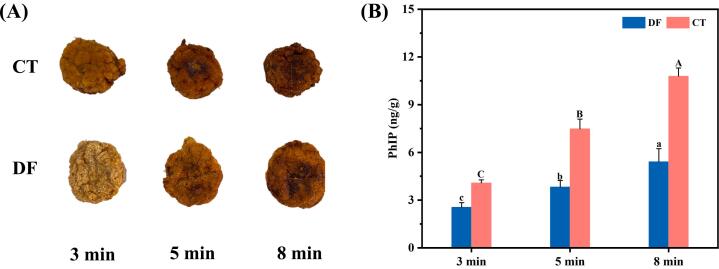
The PhIP contents in defatted golden pompano pies after pan-frying for 3, 5 and 8 min were determined and shown in Fig. 3B. As depicted, the PhIP contents in pan-fried fish pies increased with a time-dependent manner. For the CT group, the PhIP contents increased from 4.08 ng/g (3 min) to 10.79 ng/g (8 min) due to the continuous heating. In previous researches, during the roasting of pork and deep-frying of meatballs, the profound generation of PhIP were also observed (Lu, Kuhnle, & Cheng, 2018; Yan et al., 2021). While for the defatted fillets, the detected levels of PhIP were 2.55 ng/g (3 min) to 5.41 ng/g (8 min). The significant higher values of CT group than the defatted samples indicated and confirmed the contribution of lipid oxidation to the PhIP generation. Therefore, the potential promoting effect of lipid oxidation on the production of hazardous compounds like HAAs derived from protein degradation deserve more attention, in other words, the interaction and complex relationship between them on the safety of fried fish should be further investigated. In a previous work, antioxidant pomegranate peel extract was reported to inhibit the lipid oxidation effectively and consequently reduced the generation of HAAs including PhIP, IQ, Harman and Norharman during frying process of mutton meatballs (Chen et al., 2023); Plant nutmeg extracts was also observed to effectively inhibit the lipid oxidation of beef patties thus reducing the HAAs formation during the roasting processing (Parvin, Seo, Eom, Ahamed, & Yang, 2023). However, in these studies, it could only be tentatively inferred from the observations of reduced HAAs contents in meat products when lipid oxidation was inhibited. Therfore, in the current work, the contribution effect of lipid oxidation on the production of HAAs was subtly verified by the lipid removal operation, which serve as the novelty and essence. Therefore, it was logically indicated that the control and retardation of lipid oxidation in fish fillets during storage or processing by strategies like addition of antioxidants should be efficient to reduce HAAs generation in thermally processed products.
Fig. 3.
Changes in PhIP contents of defatted golden pompano pies after pan-frying for 3, 5, and 8 min. Values were mean ± standard deviation, n = 3. CT: control (without defatted fillets); DF: defatted fillets. Different lowercase letters (a-c) and uppercase letters (A-C) indicate significant difference (p < 0.05).
3.4. Changes of aldehydes contents in fish fillets during pan-frying process
The predominant aldehyde contents in pan-fried fish fillets were determined based on our established HPLC-MS/MS method. The contents of propanal, butanal, hexanal, nonanal and DDE were 4.56, 1.20, 2.58, 1.89 and 1.01 μg/g lipid in fresh sample, with other aldehydes not being detected (Table 1). After pan-frying at 180 °C for 3 min, the aldehydes contents significantly increased to 0.14, 59.38, 8.38, 32.33, 1.56, and 34.28 μg/g lipid for acrolein, propanal, crotonaldehyde, butanal, pentanal, hexanal, octanal, nonanal, DDE, HNE and HHE, respectively. However, some aldehydes continuously declined after 5 min with the extension of frying time, which indicated the superiority of the volatilization or chemical reaction of aldehydes than their generation under the extreme conditions. Numerous studies have also reported the decreased level of aldehydes in pork, and oysters with the extension of frying process (Meinert, Andersen, Bredie, Bjergegaard, & Aaslyng, 2007; Zhao, Hu, et al., 2021). These results could be attributed to the volatilization of aldehydes, and/or reaction with the nucleophilic amino group of protein or phospholipids, thus participating the chemical reaction like Maillard reaction (Echegaray et al., 2020). Moreover, the electrophilic carbonyl group of aldehydes could theoretically react with the nucleophilic amino group of PhIP and its precursors (Jing et al., 2022). Based on the abovementioned results, though the decreased aldehyde contents after 5 min, the continuously increased HAAs (PhIP) content implied the necessity to explore the specific mechanism of aldehydes on HAAs production using chemical model, which can also help to reveal the structure-activity relationship and dose-effect of aldehydes on the PhIP formation.
Table 1.
Changes of aldehyde contents (μg/g) in fish lipids during the pan-frying process.
| Compounds | Fresh | CT-3 | CT-5 | CT-8 |
|---|---|---|---|---|
| Propanal | 4.56 ± 0.18 | 59.38 ± 3.50a | 42.83 ± 1.80b | 39.07 ± 1.47b |
| Acrolein | nd | 0.14 ± 0.04b | 0.31 ± 0.01a | 0.11 ± 0.03b |
| Crotonaldehyde | nd | 3.74 ± 0.18a | 3.32 ± 0.12b | 3.11 ± 0.11b |
| Butanal | 1.20 ± 0.01 | 8.38 ± 0.96a | 7.13 ± 0.64a | 6.95 ± 0.59a |
| Pentanal | nd | 9.88 ± 0.38a | 8.78 ± 0.82a | 6.38 ± 0.74b |
| Hexanal | 2.58 ± 0.08 | 32.33 ± 3.03a | 9.75 ± 0.67b | 9.11 ± 0.37b |
| Octanal | nd | 2.42 ± 0.31c | 5.53 ± 0.77b | 8.17 ± 0.76a |
| Nonanal | 1.89 ± 0.06 | 30.06 ± 1.23a | 32.23 ± 2.37a | 30.87 ± 1.16a |
| t,t-2,4-Decadienal | 1.01 ± 0.02 | 34.28 ± 2.87a | 22.52 ± 1.23b | 17.18 ± 0.70c |
| HNE | nd | 28.3 ± 2.35a | 6.87 ± 0.37b | 6.35 ± 0.60b |
| HHE | nd | 21.29 ± 2.56a | 10.31 ± 0.27b | 7.47 ± 0.16b |
Different lowercase letters (a-c) in a row indicate significant difference from each other at 3, 5, and 8 min, respectively (p < 0.05).
3.5. Effects of aldehydes on the formation of PhIP in chemical model
To further reveal the effects and mechanism of aldehydes on the PhIP formation, various contents (0.03, 0.06, 0.12, 0.24 mmol) of aldehydes with different structures were added into the chemical model for PhIP formation. As shown in Fig. 4, the PhIP formation followed a concentration-dependent manner for the selected aldehydes and correlated with the aldehyde structure. For example, when the addition amount was 0.06 mmol, the relative contents of PhIP reached 346 %, 581 %, 148 %, 300 % and 228 %, respectively, for acrolein, propanal, hexanal, HNE and DDE compared with control group. In addition, for the propanal and hexanal, the PhIP generation was enhanced with the additive contents from 0 to 0.24 mmol. It was obviously observed that the saturated and low molecular species was more effective to exert contribution effect on HAAs generation. However, for the unsaturated aldehydes like acrolein, when the additive contents exceed 0.06 mmol, thereafter, the formation of PhIP slightly decreased (Fig. 4C-E). In line with us, previous study have found that lipid secondary oxidation products could significantly increase the formation of PhIP in model systems (Zamora, Alcón, & Hidalgo, 2012). Therefore, the lipid oxidation products are supposed to enhance the food safety risks of fried fish fillets and adversely affect consumer health after large intake. Jing et al. (2022) recently reported that lipid oxidation products acrolein effectively contribute to PhIP formation dose-dependently in a chemical model, whereas the contribution effect was attenuated when the addition of acrolein was over 0.20 mmol. From one point, aldehydes derived from lipid oxidation could facilitate the conversion of phenylalanine to phenylacetaldehyde by Strecker degradation, thus contributing to the PhIP formation (Zamora, Alcón, & Hidalgo, 2012); On the other hand, the reactive carbonyl group of acrolein allow it to react with the amino group of phenylalanine, creatinine, some intermediates of PhIP (e.g. aldol condensation product) even PhIP (Huang, Sarkhel, Roy, & Mohan, 2023; Jing et al., 2022). The evidence might be convictive to explain the observation in this study about the dose-effect of aldehydes, however, little work has revealed the structure-activity relationship under this condition. Therefore, to reveal the underlying mechanism for diverse aldehydes, the influence of aldehydes on the precursors and intermediates of PhIP should also be carefully considered.
Fig. 4.
Effects of different concentration of aldehydes addition on the PhIP formation in the chemical model. Values were mean ± standard deviation, n = 3.
3.6. Effects of aldehydes on PhIP precursors and intermediates in chemical model
The effects of aldehydes with different structures and various concentrations on PhIP formation in chemical model were determined by evaluating the contents of precursor (creatinine) and intermediate (phenylacetaldehyde) for PhIP. As demonstrated in Fig. 5A-B, the addition of propanal and DDE decreased the contents of creatinine with a concentration-dependent manner, while the content of phenylacetaldehyde gradually increased with the level of propanal and DDE until 0.06 mmol, thereafter, the content of phenylacetaldehyde decreased (Fig. 5C-D). These results indicated that the addition of low levels of propanal and DDE could promote the Strecker degradation of phenylalanine to generate more phenylacetaldehyde, which condenses with more creatinine to produce aldol condensation product, eventually more PhIP formation. However, propanal and DDE might react with precursors and PhIP when the concentration of them more than 0.06 mmol, resulting in the decreased of PhIP formation, theoretically, the strong electrophilic properties of aldehydes could react with the amino group of amino acid, creatinine, even PhIP (Jing et al., 2022). For other aldehydes like acrolein and HNE, similar trend for the contents of phenylacetaldehyde and creatinine was not observed due to their reactivity toward amine groups, which also indicated the complexity between lipid oxidation and HAAs production during food processing.
Fig. 5.
Effects of different concentration of aldehydes on the contents of creatinine and phenylacetaldehyde in the chemical model. Values were mean ± standard deviation, n = 3.
4. Conclusions
The TBARS value increasement from lipid oxidation significantly contributed to the PhIP generation during pan-frying process of golden pompano fillets. Furthermore, the defat treatment of fish meat could significantly reduce the PhIP formation during pan-frying process. Lipid oxidation products aldehydes could congruously promote the PhIP formation with the dose-effect and structure-activity relationship by converting phenylalanine to phenylacetaldehyde by Strecker degradation. Unsaturated and low molecular species was more effective to exert the contribution effect on HAAs generation. The contribution effect was slightly decreased with high levels of reactive aldehydes addition due to their strong electrophilic properties and the participation of reaction with the amino group of phenylacetaldehyde, creatinine, even PhIP.
CRediT authorship contribution statement
Mantong Zhao: Writing – original draft, Visualization, Software, Methodology, Investigation, Data curation, Conceptualization. Zhongyuan Liu: Writing – review & editing, Supervision, Conceptualization. Mengyin Zha: Formal analysis, Conceptualization. Ying Sun: Formal analysis, Conceptualization. Haohao Shi: Methodology, Conceptualization. Xueying Zhang: Writing – review & editing, Conceptualization. Chuan Li: Supervision, Conceptualization. Guanghua Xia: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by Natural Science Foundation of China (32402196), Natural Science Foundation of Hainan Province (322RC585), Primary Research & Development Plan of Hainan Province (ZDYF2022XDNY335), Science and Technology Project of Haikou City (2023–044), and Fund of Key Laboratory of Aquatic Product Processing, Ministry of Agriculture and Rural Affairs, China (NYJG202302).
Data availability
Data will be made available on request.
References
- Alaejos M.S., Afonso A.M. Factors that affect the content of heterocyclic aromatic amines in foods. Comprehensive Reviews in Food Science and Food Safety. 2011;10(2):52–108. doi: 10.1111/j.1541-4337.2010.00141.x. [DOI] [Google Scholar]
- Bellamri M., Brandt K., Cammerrer K., Syeda T., Turesky R.J., Cannon J.R. Nuclear DNA and mitochondrial damage of the cooked meat carcinogen 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine in human neuroblastoma cells. Chemical Research in Toxicology. 2023;36(8):1361–1373. doi: 10.1021/acs.chemrestox.3c00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Shi X., Zhao R., Tian Z., Zhang L., Yu Q., Chen C. Effect of pomegranate peel extract on the reduction of heterocyclic amines and quality characteristics of mutton meatballs. Food Quality and Safety. 2023;7 doi: 10.1093/fqsafe/fyac073. [DOI] [Google Scholar]
- Chen X., Jia W., Zhu L., Mao L., Zhang Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Comprehensive Reviews in Food Science and Food Safety. 2020;19(1):124–148. doi: 10.1111/1541-4337.12511. [DOI] [PubMed] [Google Scholar]
- Echegaray N., Paterio M., Domínguez R., Purriños L., Bermúdez R., Carballo J., Lorenzo J.M. Effects of different cooking methods and of the inclusion of chestnut (Castanea sativa miller) in the finishing diet of Celta pig breed on the physicochemical parameters and volatile profile of longissimus thoracis et lumborum muscle. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109407. [DOI] [PubMed] [Google Scholar]
- Gao C., Zhao M., Wang X., Wang J., Li C., Dong X., Liu Z., Zhou D. Plasma-activated water in combination with coconut exocarp flavonoids emerge as promising preservation technique for golden pompano: Impact of the treatment sequence. Food Chemistry. 2024;447 doi: 10.1016/j.foodchem.2024.138981. [DOI] [PubMed] [Google Scholar]
- Geng Y., Xie Y., Li W., Ji J., Chen F., Liao X., Hu X., Ma L. Heterocyclic amines in meat and meat products: Occurrence, formation, mitigation, health risks and intervention. Food Reviews International. 2024;40(5):1503–1519. doi: 10.1080/87559129.2023.2221346. [DOI] [Google Scholar]
- Gertz C., Aladedunye F., Matthäus B. Oxidation and structural decomposition of fats and oils at elevated temperatures. European Journal of Lipid Science and Technology. 2014;116(11):1457–1466. doi: 10.1002/ejlt.201400099. [DOI] [Google Scholar]
- Gibis M. Heterocyclic aromatic amines in cooked meat products: Causes, formation, occurrence, and risk assessment. Comprehensive Reviews in Food Science and Food Safety. 2016;15(2):269–302. doi: 10.1111/1541-4337.12186. [DOI] [PubMed] [Google Scholar]
- Hidalgo F.J., Zamora R. Carbonyl chemistry and the formation of heterocyclic aromatic amines with the structure of aminoimidazoazaarene. Journal of Agricultural and Food Chemistry. 2021;70(1):79–86. doi: 10.1021/acs.jafc.1c06842. [DOI] [PubMed] [Google Scholar]
- Huang Y., Sarkhel S., Roy A., Mohan A. Interrelationship of lipid aldehydes (MDA, 4-HNE, and 4-ONE) mediated protein oxidation in muscle foods. Critical Reviews in Food Science and Nutrition. 2023:1–17. doi: 10.1080/10408398.2023.2245029. [DOI] [PubMed] [Google Scholar]
- Iarc I.A.R.C. International Agency for Research on Cancer; Lyon: 1993. Some naturally occurring aromatic amines and mycotoxins. [Google Scholar]
- Jiang Y., Jiang Q., Fan D., Wang M., Zhao Y. Effect of Acrolein, a lipid oxidation product, on the formation of the heterocyclic aromatic amine 2-Amino-3, 8-dimethylimidazo [4, 5-f] quinoxaline (MeIQx) in model systems and roast Salmon patties. Journal of Agricultural and Food Chemistry. 2022;70(19):5887–5895. doi: 10.1021/acs.jafc.2c00970. [DOI] [PubMed] [Google Scholar]
- Jing M., Jiang Q., Zhu Y., Fan D., Wang M., Zhao Y. Effect of acrolein, a lipid oxidation product, on the formation of the heterocyclic aromatic amine 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) in model systems and roasted tilapia fish patties. Food Chemistry: X. 2022;14 doi: 10.1016/j.fochx.2022.100315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.J., Lee S.Y., Kang J.H., Kim J.H., Kim H.W., Oh D.H.…Hur S.J. Main mechanisms for carcinogenic heterocyclic amine reduction in cooked meat by natural materials. Meat Science. 2022;183 doi: 10.1016/j.meatsci.2021.108663. [DOI] [PubMed] [Google Scholar]
- Lu F., Kuhnle G.K., Cheng Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control. 2018;92:399–411. doi: 10.1016/j.foodcont.2018.05.018. [DOI] [Google Scholar]
- Ma L., Liu G., Liu X. Amounts of malondialdehyde do not accurately represent the real oxidative level of all vegetable oils: A kinetic study of malondialdehyde formation. International Journal of Food Science & Technology. 2019;54(2):412–423. doi: 10.1111/ijfs.13952. [DOI] [Google Scholar]
- Meinert L., Andersen L.T., Bredie W.L., Bjergegaard C., Aaslyng M.D. Chemical and sensory characterisation of pan-fried pork flavour: Interactions between raw meat quality, ageing and frying temperature. Meat Science. 2007;75(2):229–242. doi: 10.1016/j.meatsci.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Parvin R., Seo J.K., Eom J.U., Ahamed Z., Yang H.S. Inhibitory and antioxidative capacity of nutmeg extracts on reduction of lipid oxidation and heterocyclic amines in pan-roasted beef patties. Meat Science. 2023;197 doi: 10.1016/j.meatsci.2022.109064. [DOI] [PubMed] [Google Scholar]
- Shahidi F., Hossain A. Role of lipids in food flavor generation. Molecules. 2022;27(15):5014. doi: 10.3390/molecules27155014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Chu X., Du P., He H., He F., Liu Y.…Abd El-Aty A.M. Unveiling heterocyclic aromatic amines (HAAs) in thermally processed meat products: Formation, toxicity, and strategies for reduction–a comprehensive review. Food Chemistry: X. 2023 doi: 10.1016/j.fochx.2023.100833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Liu Z., Zhao M., Gao C., Wang J., Li C., Dong X., Liu Z., Zhou D. Chitosan-wampee seed essential oil composite film combined with cold plasma for refrigerated storage with modified atmosphere packaging: A promising technology for quality preservation of golden pompano fillets. International Journal of Biological Macromolecules. 2023;224:1266–1275. doi: 10.1016/j.ijbiomac.2022.10.212. [DOI] [PubMed] [Google Scholar]
- Xu L., Mei X., Wu G., Karrar E., Jin Q., Wang X. Inhibitory effect of antioxidants on key off-odors in French fries and oils and prolong the optimum frying stage. LWT. 2022;162 doi: 10.1016/j.lwt.2022.113417. [DOI] [Google Scholar]
- Xu Y., Li H., Liang J., Ma J., Yang J., Zhao X., Zhao W., Bai W., Zeng X., Dong H. High-throughput quantification of eighteen heterocyclic aromatic amines in roasted and pan-fried meat on the basis of high performance liquid chromatography-quadrupole-orbitrap high resolution mass spectrometry. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130147. [DOI] [PubMed] [Google Scholar]
- Xu Y., Ye H., Dong H., Zeng X., Yang J., Xiao G., Bai W., Wu J., He Q., Xian Y. Determination of 2-amino-1-methyl-6-phenylimidazole [4, 5-b] pyridine (PhIP) and its precursors and possible intermediates in a chemical model system and roast pork. LWT. 2023;177 doi: 10.1016/j.lwt.2023.114581. [DOI] [Google Scholar]
- Yan Y., You F.H., Zeng M.M., Chen J., Huang J.J., Jiang J. Evaluating the effects of temperature and time on heterocyclic aromatic amine profiles in roasted pork using combined UHPLC-MS/MS and multivariate analysis. Food Research International. 2021;141 doi: 10.1016/j.foodres.2021.110134. [DOI] [PubMed] [Google Scholar]
- Yang H., Ji Z., Wang R., Fan D., Zhao Y., Wang M. Inhibitory effect of selected hydrocolloids on 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) formation in chemical models and beef patties. Journal of Hazardous Materials. 2021;402 doi: 10.1016/j.jhazmat.2020.123486. [DOI] [PubMed] [Google Scholar]
- Zamora R., Alcón E., Hidalgo F.J. Effect of lipid oxidation products on the formation of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) in model systems. Food Chemistry. 2012;135(4):2569–2574. doi: 10.1016/j.foodchem.2012.06.062. [DOI] [PubMed] [Google Scholar]
- Zamora R., Delgado R.M., Hidalgo F.J. Chemical conversion of phenylethylamine into phenylacetaldehyde by carbonyl-amine reactions in model systems. Journal of Agricultural and Food Chemistry. 2012;60(21):5491–5496. doi: 10.1021/jf301258s. [DOI] [PubMed] [Google Scholar]
- Zamora R., Hidalgo F.J. 2-Amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) formation and fate: An example of the coordinate contribution of lipid oxidation and Maillard reaction to the production and elimination of processing-related food toxicants. RSC Advances. 2015;5(13):9709–9721. doi: 10.1039/C4RA15371E. [DOI] [Google Scholar]
- Zhang H., Lv X., Su W., Chen B.H., Lai Y.W., Xie R.…Cao H. Exploring the roles of excess amino acids, creatine, creatinine, and glucose in the formation of heterocyclic aromatic amines by UPLC-MS/MS. Food Chemistry. 2024;446 doi: 10.1016/j.foodchem.2024.138760. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cao J., Pei Z., Wei P., Xiang D., Cao X., Shen X., Li C. Volatile flavour components and the mechanisms underlying their production in golden pompano (Trachinotus blochii) fillets subjected to different drying methods: A comparative study using an electronic nose, an electronic tongue and SDE-GC-MS. Food Research International. 2019;123:217–225. doi: 10.1016/j.foodres.2019.04.069. [DOI] [PubMed] [Google Scholar]
- Zhao G.H., Hu Y.Y., Liu Z.Y., Xie H.K., Zhang M., Zheng R.…Zhou D.Y. Simultaneous quantification of 24 aldehydes and ketones in oysters (Crassostrea gigas) with different thermal processing procedures by HPLC-electrospray tandem mass spectrometry. Food Research International. 2021;147 doi: 10.1016/j.foodres.2021.110559. [DOI] [PubMed] [Google Scholar]
- Zhao M., Liu Z., Sun Y., Shi H., Yun Y., Zhao M., Xia G., Shen X. Novel hydrocolloids synthesized by polyphenols grafted onto chitosan: A promising coating to inhibit PhIP formation during pan-frying of golden pompano fillets. Food Chemistry. 2024;447 doi: 10.1016/j.foodchem.2024.139029. [DOI] [PubMed] [Google Scholar]
- Zhao M., Liu Z., Zhao Y., Gao C., Wang J., Xia G., Li C., Zhou D. Dielectric barrier discharge cold plasma collaborated with coconut exocarp flavonoids: A promising technology for oyster preservation under refrigerated storage. LWT. 2024 doi: 10.1016/j.lwt.2024.115888. [DOI] [Google Scholar]
- Zhao X., Wu Y., Liu H., Hu N., Zhang Y., Wang S. Grape seed extract ameliorates PhIP-induced colonic injury by modulating gut microbiota, lipid metabolism, and NF-κB signaling pathway in rats. Journal of Functional Foods. 2021;78 doi: 10.1016/j.jff.2021.104362. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.