Abstract
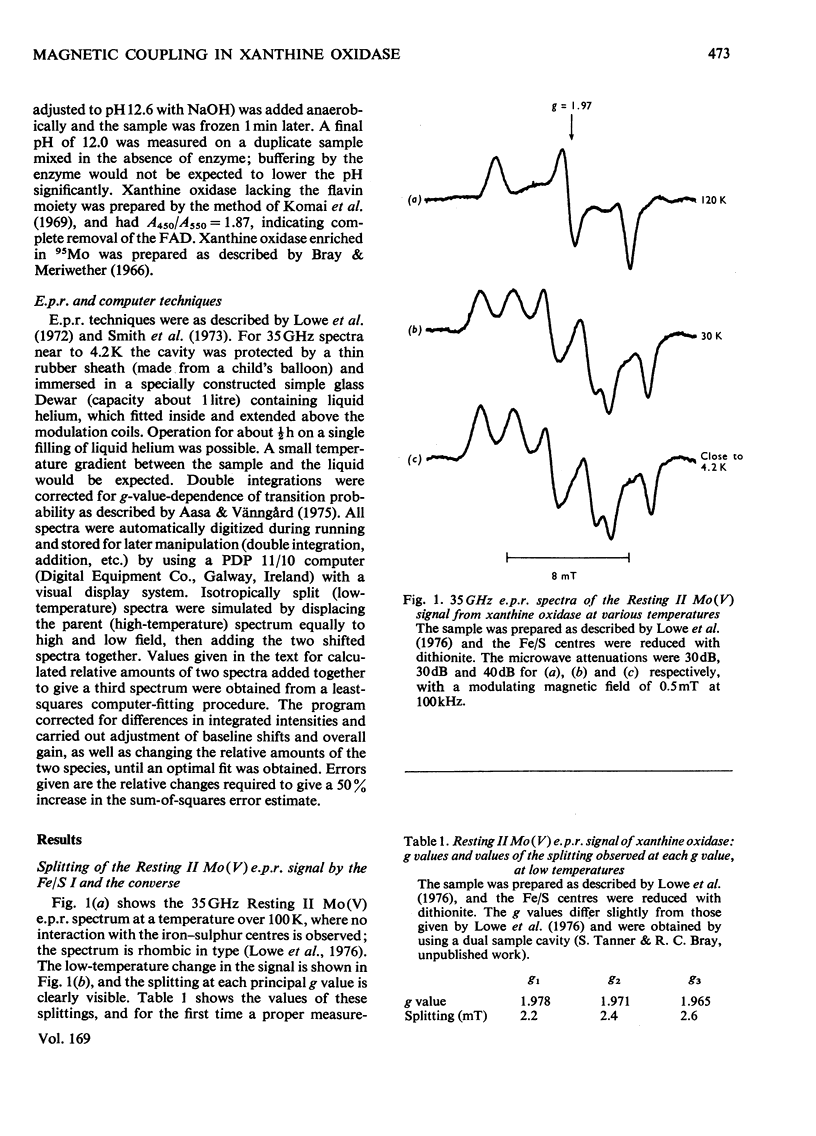
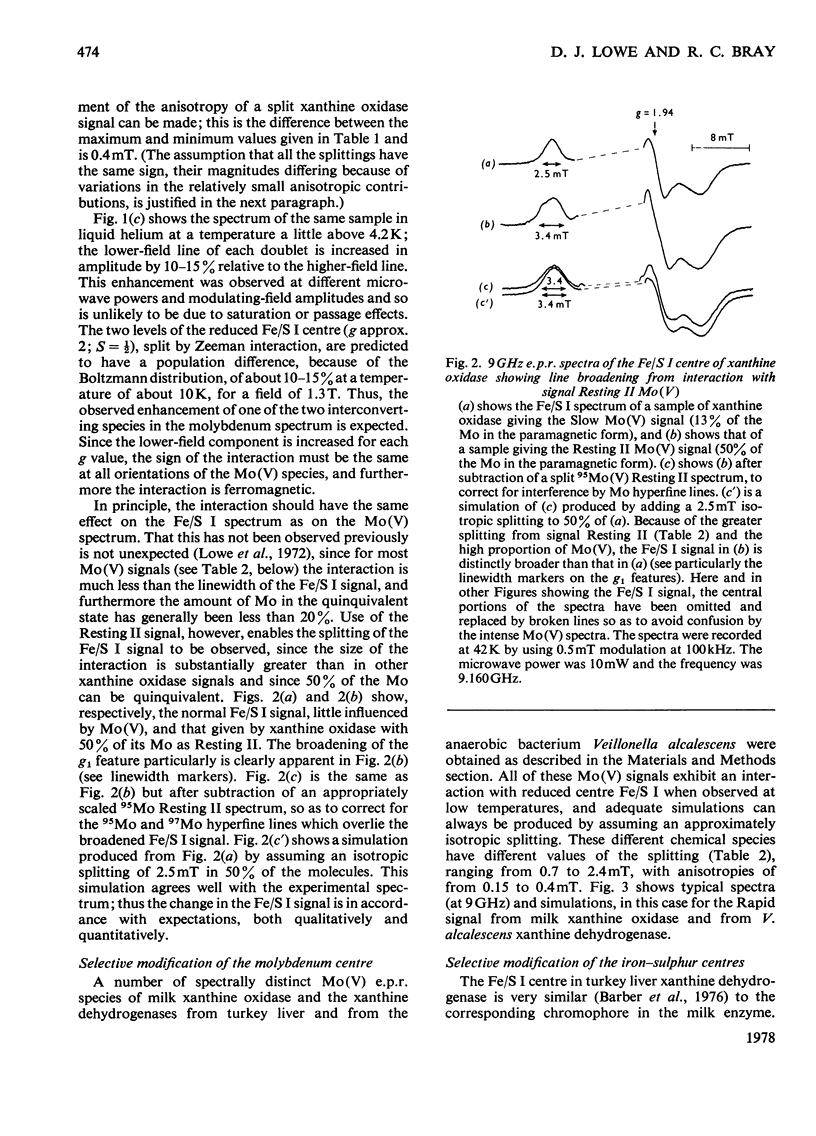
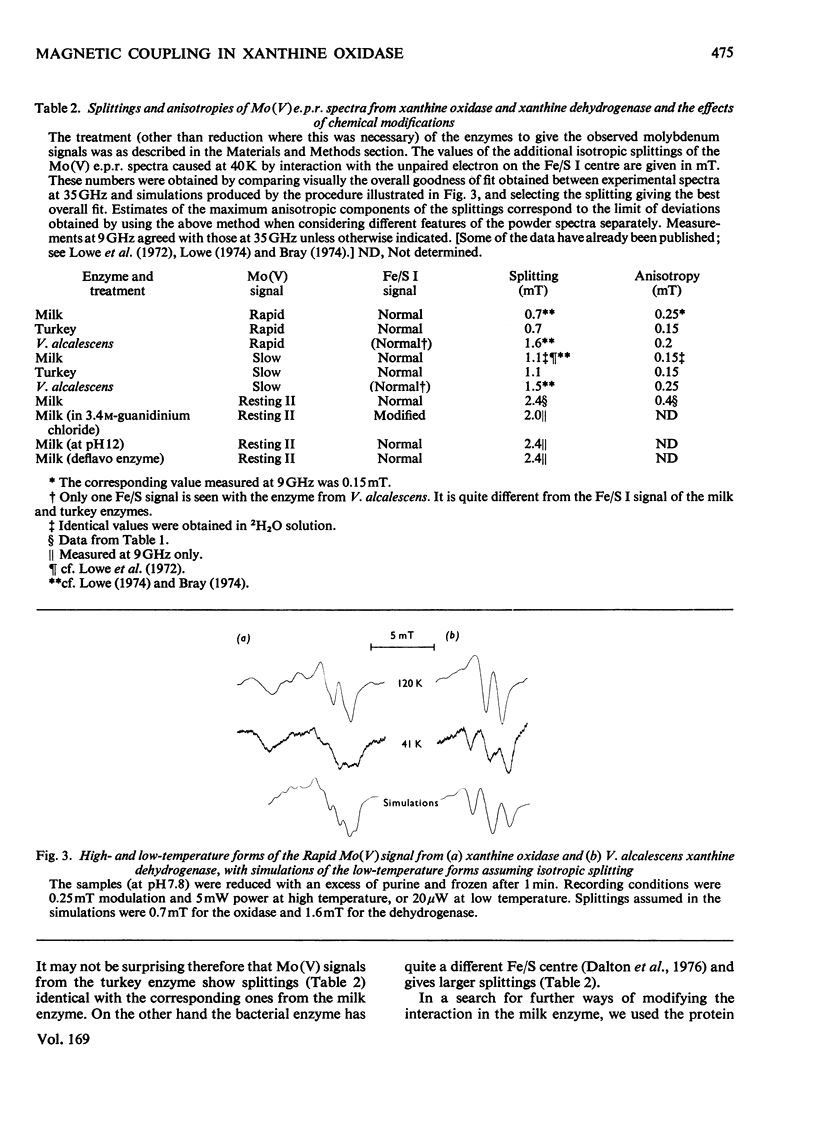
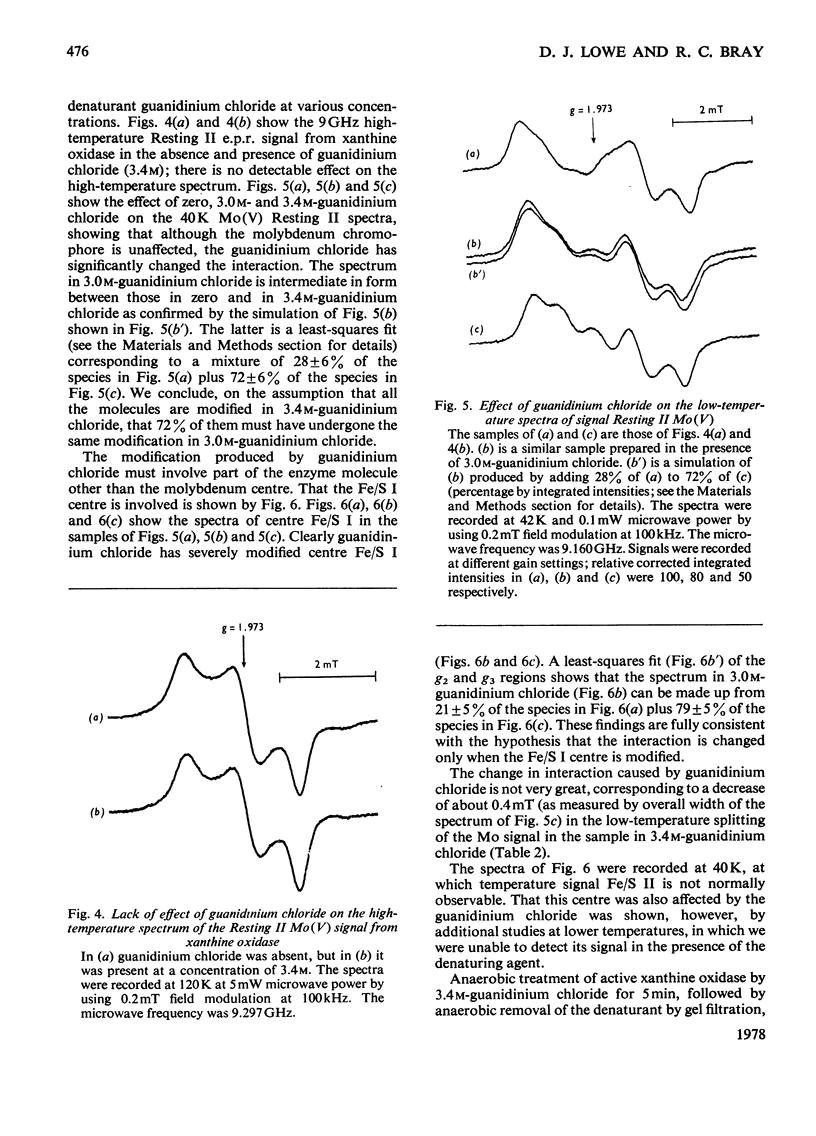
Magnetic interaction between molybdenum and one of the iron-sulphur centres in milk xanthine oxidase [Lowe, Lynden-Bell & Bray (1972) Biochem. J. 130, 239-249] was studied further, with particular reference to the newly discovered Mo(V) e.p.r.(electron-paramagnetic-resonance) signal, Resting II [Lowe, Barber, Pawlik & Bray (1976) Biochem. J. 155, 81-85]. E.p.r. measurements at 35GHz near to 4.2K showed that the interaction has the same sign at all molybdenum orientations and is ferromagnetic. The predicted splitting of the e.p.r. signal from the reduced iron-sulphur centre, Fe/S I, was observed, Providing positive identification of this as the other interacting species. Chemical modification of the molybdenum environment in xanthine oxidase can change the size of the interaction severalfold, but interaction always remains approximately isotropic. The interaction in turkey liver xanthine dehydrogenase is indistinguishable from that in the oxidase. However, a bacterial xanthine dehydrogenase with different iron-sulphur centres shows rather larger interaction. Guanidinium chloride disturbs the iron-sulphur centres of the oxidase, and when this occurs there is a parallel and relatively small change in the interaction. Removal of flavin from the molecule, or raising the pH to 12.0, changes the interaction slightly without affecting the chromophores themselves. It is concluded that the Fe/S I centre and the Mo are at least 1.0nm and probably nearer 2.5nm apart, and that the conformation of the protein between them is relatively stable up to pH 12.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber M. J., Bray R. C., Lowe D. J., Coughlan M. P. Studies by electron-paramagnetic-resonance spectroscopy and stopped-flow spectrophotometry on the mechanism of action of turkey liver xanthine dehydrogenase. Biochem J. 1976 Feb 1;153(2):297–307. doi: 10.1042/bj1530297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray R. C., Meriwether L. S. Electron spin resonance of xanthine oxidase substituted with molybdenum-95. Nature. 1966 Oct 29;212(5061):467–469. doi: 10.1038/212467a0. [DOI] [PubMed] [Google Scholar]
- Cleere W. F., Coughlan M. P. Avian xanthine dehydrogenases. I. Isolation and characterization of the turkey liver enzyme. Comp Biochem Physiol B. 1975 Feb 15;50(2B):311–322. doi: 10.1016/0305-0491(75)90280-1. [DOI] [PubMed] [Google Scholar]
- Dalton H., Lowe D. J., Pawlik T., Bray R. C. Studies by electron-paramagnetic-resonance spectroscopy on the mechanism of action of xanthine dehydrogenase from Veillonella alcalescens. Biochem J. 1976 Feb 1;153(2):287–295. doi: 10.1042/bj1530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart L. I., McGartoll M. A., Chapman H. R., Bray R. C. The composition of milk xanthine oxidase. Biochem J. 1970 Mar;116(5):851–864. doi: 10.1042/bj1160851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Salerno J. C., Ohnishi T. Studies on electron paramagnetic resonance spectra manifested by a respiratory chain hydrogen carrier. Arch Biochem Biophys. 1976 Nov;177(1):176–184. doi: 10.1016/0003-9861(76)90427-6. [DOI] [PubMed] [Google Scholar]
- Komai H., Massey V., Palmer G. The preparation and properties of deflavo xanthine oxidase. J Biol Chem. 1969 Apr 10;244(7):1692–1700. [PubMed] [Google Scholar]
- Lowe D. J., Barber M. J., Pawlik R. T., Bray R. C. A new non-functional form of milk xanthine oxidase containing stable quinquivalent molybdenum. Biochem J. 1976 Apr 1;155(1):81–85. doi: 10.1042/bj1550081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. J., Hyde J. S. Electron-electron double resonance measurements on xanthine oxidase. Biochim Biophys Acta. 1975 Jan 23;377(1):205–210. doi: 10.1016/0005-2744(75)90302-2. [DOI] [PubMed] [Google Scholar]
- Lowe D. J., Lynden-Bell R. M., Bray R. C. Spin-spin interaction between molybdenum and one of the iron-sulphur systems of xanthine oxidase and its relevance to the enzymic mechanism. Biochem J. 1972 Nov;130(1):239–249. doi: 10.1042/bj1300239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Mathews R., Charlton S., Sands R. H., Palmer G. On the nature of the spin coupling between the iron-sulfur clusters in the eight-iron ferredoxins. J Biol Chem. 1974 Jul 10;249(13):4326–4328. [PubMed] [Google Scholar]
- Pick F. M., Bray R. C. Complex-formation between reduced xanthine oxidase and purine substrates demonstrated by electron paramagnetic resonance. Biochem J. 1969 Oct;114(4):735–742. doi: 10.1042/bj1140735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H., Schepler K. L., Dunham W. R., Sands R. H. Interaction of ubisemiquinone with a paramagnetic component in heart tissue. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2886–2890. doi: 10.1073/pnas.72.8.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepler K. L., Dunham W. R., Sands R. H., Fee J. A., Abeles R. H. A physical explanation of the EPR spectrum observed during catalysis by enzymes utilizing coenzyme B12. Biochim Biophys Acta. 1975 Aug 26;397(2):510–518. doi: 10.1016/0005-2744(75)90141-2. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Lowe D. J., Bray R. C. Studies by electron paramagnetic resonance on the catalytic mechanism of nitrogenase of Klebsiella pneumoniae. Biochem J. 1973 Oct;135(2):331–341. doi: 10.1042/bj1350331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Van Heuvelen A. Electron transport in xanthine oxidase. A model for other biological electron transport chains. Biophys J. 1976 Aug;16(8):939–951. doi: 10.1016/S0006-3495(76)85744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


