Abstract
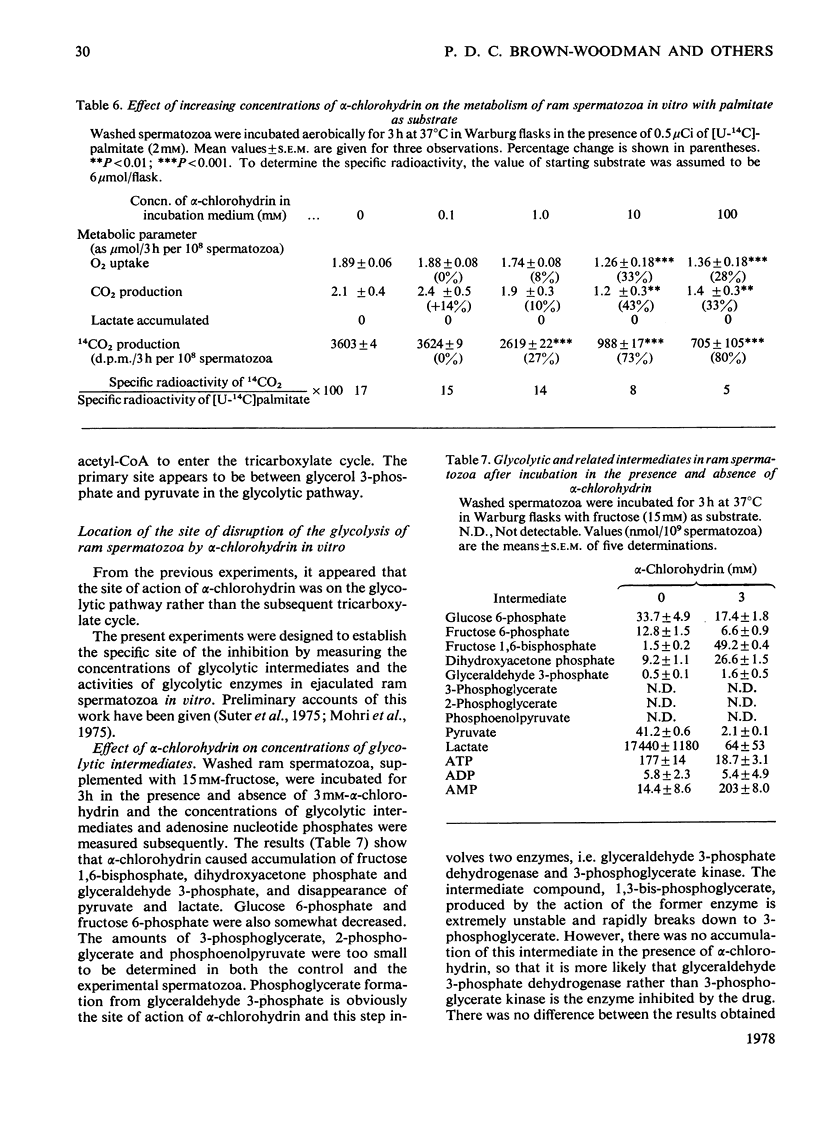
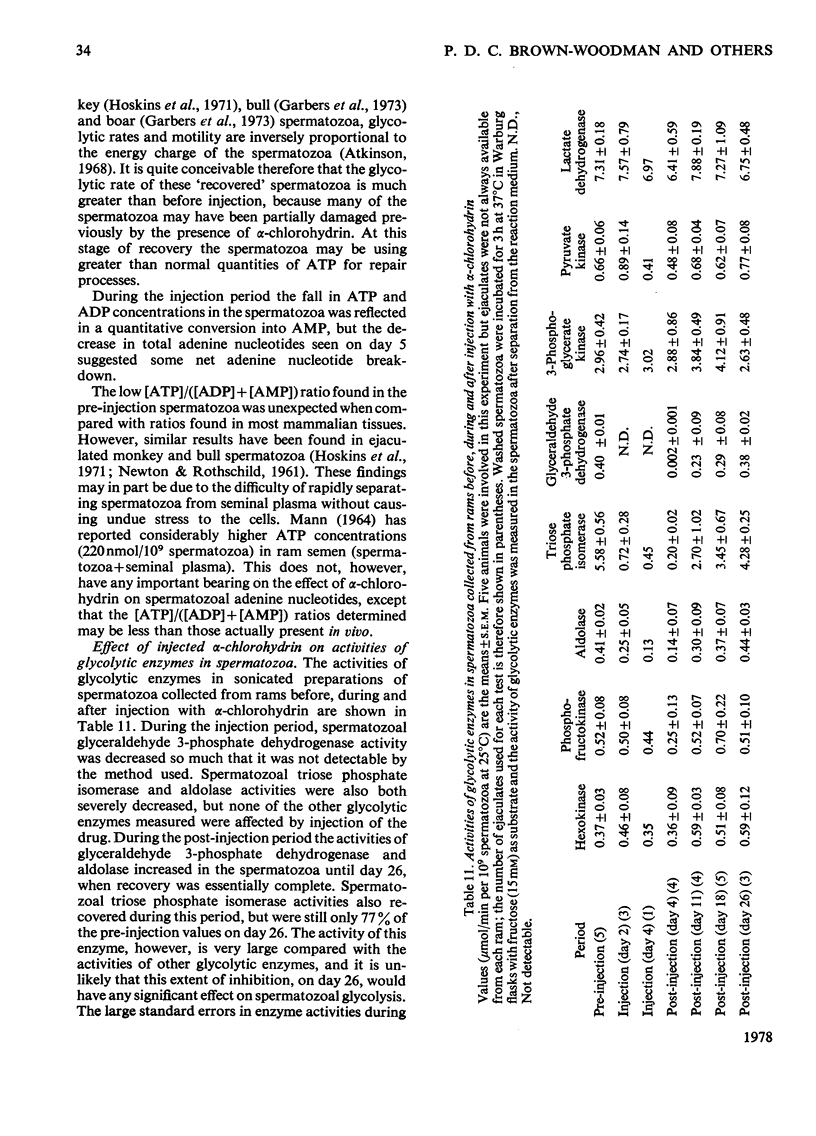
1. The effect of α-chlorohydrin on the metabolism of glycolytic and tricarboxylate-cycle substrates by ram spermatozoa was investigated. The utilization and oxidation of fructose and triose phosphate were much more sensitive to inhibition by α-chlorohydrin (0.1–1.0mm) than lactate or pyruvate. Inhibition of glycolysis by α-chlorohydrin is concluded to be between triose phosphate and pyruvate formation. Oxidation of glycerol was not as severely inhibited as that of the triose phosphate. This unexpected finding can be explained in terms of competition between glycerol and α-chlorohydrin. A second, much less sensitive site, of α-chlorohydrin inhibition appears to be associated with production of acetyl-CoA from exogenous and endogenous fatty acids. 2. Measurement of the glycolytic intermediates after incubation of spermatozoal suspensions with 15mm-fructose in the presence of 3mm-α-chlorohydrin showed a `block' in the conversion of glyceraldehyde 3-phosphate into 3-phosphoglycerate. α-Chlorohydrin also caused conversion of most of the ATP in spermatozoa into AMP. After incubation with 3mm-α-chlorohydrin, glyceraldehyde 3-phosphate dehydrogenase and triose phosphate isomerase activities were decreased by approx. 90% and 80% respectively, and in some experiments aldolase was also inhibited. Other glycolytic enzymes were not affected by a low concentration (0.3mm) of α-chlorohydrin. Loss of motility of spermatozoa paralleled the decrease in glyceraldehyde 3-phosphate dehydrogenase activity. α-Chlorohydrin, however, did not inhibit glyceraldehyde 3-phosphate dehydrogenase or triose phosphate isomerase in sonicated enzyme preparations when added to the assay cuvette. 3. Measurement of intermediates and glycolytic enzymes in ejaculated spermatozoa before, during and after injection of rams with α-chlorohydrin (25mg/kg body wt.) confirmed a severe block in glycolysis in vivo at the site of triose phosphate conversion into 3-phosphoglycerate within 24h of the first injection. Glyceraldehyde 3-phosphate dehydrogenase activity was no longer detectable and both aldolase and triose phosphate isomerase were severely inhibited. Spermatozoal ATP decreased by 92% at this time, being quantitatively converted into AMP. At 1 month after injection of α-chlorohydrin glycolytic intermediate concentrations returned to normal in the spermatozoa but ATP was still only 38% of the pre-injection concentration. Motility of spermatozoa was, however, as good as during the pre-injection period. The activity of the inhibited enzymes also returned to normal during the recovery period and 26 days after injection were close to pre-injection values. 4. An unknown metabolic product of α-chlorohydrin is suggested to inhibit glyceraldehyde 3-phosphate dehydrogenase and triose phosphate isomerase of spermatozoa. This results in a lower ATP content, motility and fertility of the spermatozoa. Glycidol was shown not to be an active intermediate of α-chlorohydrin in vitro.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- BUBLITZ C., KENNEDY E. P. Synthesis of phosphatides in isolated mitochondria. III. The enzymatic phosphorylation of glycerol. J Biol Chem. 1954 Dec;211(2):951–961. [PubMed] [Google Scholar]
- Brown-Woodman P. D., Salamon S., White I. G. Effect of alpha-chlorohydrin on the fertility of rams. Acta Eur Fertil. 1974 Sep;5(3):193–197. [PubMed] [Google Scholar]
- Brown-Woodman P. D., Sale D., White I. G. The glycerylphosphorylcholine content of the rat epididymis after injecting alpha-chlorohydrin and ligating the vasa efferentia. Acta Eur Fertil. 1976 Jun;7(2):155–162. [PubMed] [Google Scholar]
- Brown-Woodman P. D., White I. G. A comparison of the inhibition of the metabolism of ram and rabbit spermatozoa be alpha-chlorohydrin in vitro. Theriogenology. 1976 Jul;6(1):29–37. doi: 10.1016/0093-691x(76)90185-0. [DOI] [PubMed] [Google Scholar]
- Brown-Woodman P. D., White I. G. Disruption of the metabolism, motility and morphology of spermatozoa by injection of alpha-chlorohydrin into rams. Aust J Biol Sci. 1976 Dec;29(5-6):545–555. doi: 10.1071/bi9760545. [DOI] [PubMed] [Google Scholar]
- Brown-Woodman P. D., White I. G., Salamon S. Proceedings: effect of alpha-chlorohydrin on the fertility of rams and on the metabolism of spermatozoa in vitro. J Reprod Fertil. 1975 May;43(2):381–381. doi: 10.1530/jrf.0.0430381. [DOI] [PubMed] [Google Scholar]
- Brown P. D., White I. G. Studies on the male anti-fertility drug 3-chloro-1,2-propanediol. J Reprod Fertil. 1973 Feb;32(2):337–338. doi: 10.1530/jrf.0.0320337. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Chulavatnatol M., Hasibuan I., Yindepit S., Eksittikul T. Lack of effect of alpha-chlorohydrin on the ATP content of rat, mouse and human spermatozoa. J Reprod Fertil. 1977 May;50(1):137–139. doi: 10.1530/jrf.0.0500137. [DOI] [PubMed] [Google Scholar]
- Coppola J. A. An extragonadal male antifertility agent. Life Sci. 1969 Jan 1;8(1):43–48. doi: 10.1016/0024-3205(69)90291-4. [DOI] [PubMed] [Google Scholar]
- Crabo B., Appelgren L. E. Distribution of ( 14 C) -chlorohydrin in mice and rats. J Reprod Fertil. 1972 Jul;30(1):161–163. doi: 10.1530/jrf.0.0300161. [DOI] [PubMed] [Google Scholar]
- Edwards E. M., Jones A. R., Waites G. M. The entry of alpha-chlorohydrin into body fluids of male rats and its effect upon the incorporation of glycerol into lipids. J Reprod Fertil. 1975 May;43(2):225–232. doi: 10.1530/jrf.0.0430225. [DOI] [PubMed] [Google Scholar]
- Ericsson R. J., Baker V. F. Male antifertility compounds: biological properties of U-5897 and U-l5,646. J Reprod Fertil. 1970 Mar;21(2):267–273. doi: 10.1530/jrf.0.0210267. [DOI] [PubMed] [Google Scholar]
- Ericsson R. J. Male antifertility compounds: U-5897 as a rat chemosterilant. J Reprod Fertil. 1970 Jul;22(2):213–222. doi: 10.1530/jrf.0.0220213. [DOI] [PubMed] [Google Scholar]
- Garbers D. L., First N. L., Gorman S. K., Lardy H. A. The effects of cyclic nucleotide phosphodiesterase inhibitors on ejaculated porcine spermatozoan metabolism. Biol Reprod. 1973 Jun;8(5):599–606. doi: 10.1093/biolreprod/8.5.599. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., White I. G. Glycolytic enzymes in the spermatozoa and cytoplasmic droplets of bull, boar and ram, and their leakage afte shock. J Reprod Fertil. 1972 Jul;30(1):105–115. doi: 10.1530/jrf.0.0300105. [DOI] [PubMed] [Google Scholar]
- Hodgen G. D., Butler W. R., Gomes W. R. In vivo and in vitro effects of cadmium chloride on carbonic anhydrase activity. J Reprod Fertil. 1969 Feb;18(1):156–157. doi: 10.1530/jrf.0.0180156. [DOI] [PubMed] [Google Scholar]
- Hoskins D. D., Stephens D. T., Casillas E. R. Enzymic control of fructolysis in primate spermatozoa. Biochim Biophys Acta. 1971 May 18;237(2):227–238. doi: 10.1016/0304-4165(71)90314-x. [DOI] [PubMed] [Google Scholar]
- Jackson H., Campbell I. S., Jones A. R. Is glycidol an active intermediate in the antifertility action of alpha-chlorhydrin in male rats? Nature. 1970 Apr 4;226(5240):86–87. doi: 10.1038/226086a0. [DOI] [PubMed] [Google Scholar]
- Jones A. R., Davies P., Edwards K., Jackson H. Antifertility effects and metabolism of alpha and epi-chlorhydrins in the rat. Nature. 1969 Oct 4;224(5214):83–83. doi: 10.1038/224083a0. [DOI] [PubMed] [Google Scholar]
- Kreider J. L., Dutt R. H. Induction of temporary infertility in rams with an orally administered chlorohydrin. J Anim Sci. 1970 Jul;31(1):95–98. doi: 10.2527/jas1970.31195x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MOHRI H., MOHRI T., ERNSTER L. ISOLATI N AND ENZYMIC PROPERTIES OF THE MIDPIECE OF BULL SPERMATOZOA. Exp Cell Res. 1965 May;38:217–246. doi: 10.1016/0014-4827(65)90400-3. [DOI] [PubMed] [Google Scholar]
- Mohri H., Suter D. A., Brown-Woodman P. D., White I. G., Ridley D. D. Identification of the biochemical lesion produced by alpha-chlorohydrin in spermatozoa. Nature. 1975 May 1;255(5503):75–77. doi: 10.1038/255075a0. [DOI] [PubMed] [Google Scholar]
- Samojlik E., Chang M. C. Antifertility activity of 3-chloro-1, 2-propanediol (U-5897) on male rats. Biol Reprod. 1970 Apr;2(2):299–304. doi: 10.1095/biolreprod2.2.299. [DOI] [PubMed] [Google Scholar]
- Suter D. A., Brown-Woodman P. D., Mohri H., White I. G. Proceedings: the molecular site of action of the anti-fertility agent, alpha-chlorohydrin, in ram spermatozoa. J Reprod Fertil. 1975 May;43(2):382–383. doi: 10.1530/jrf.0.0430382. [DOI] [PubMed] [Google Scholar]
- Vickery B. H., Erickson G. I., Bennett J. P. Mechanism of antifertility action of low doses of alpha-chlorohydrin in the male rat. J Reprod Fertil. 1974 May;38(1):1–10. doi: 10.1530/jrf.0.0380001. [DOI] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- White I. G., Bronw-Woodman P. D., Suter D. A., Mohri H. Proceedings: Inhibition of the oxidative metabolism of ram spermatozoa by alpha-chlorohydrin in vitro. J Reprod Fertil. 1976 Mar;46(2):509–509. doi: 10.1530/jrf.0.0460509-a. [DOI] [PubMed] [Google Scholar]
- White I. G. Effect of alpha-chlorohydrin on cauda epididymis and spermatozoa of the rat and general physiological status. Contraception. 1975 Jan;11(1):69–77. doi: 10.1016/0010-7824(75)90052-9. [DOI] [PubMed] [Google Scholar]


