Abstract
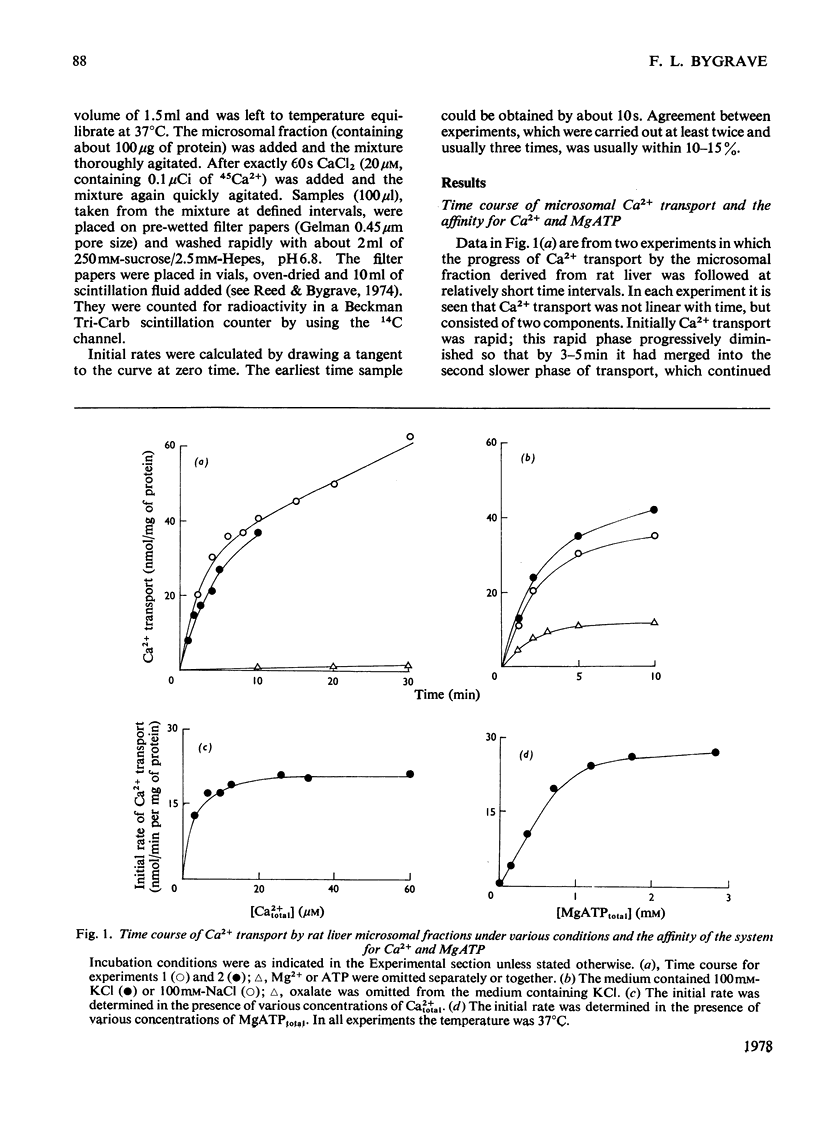
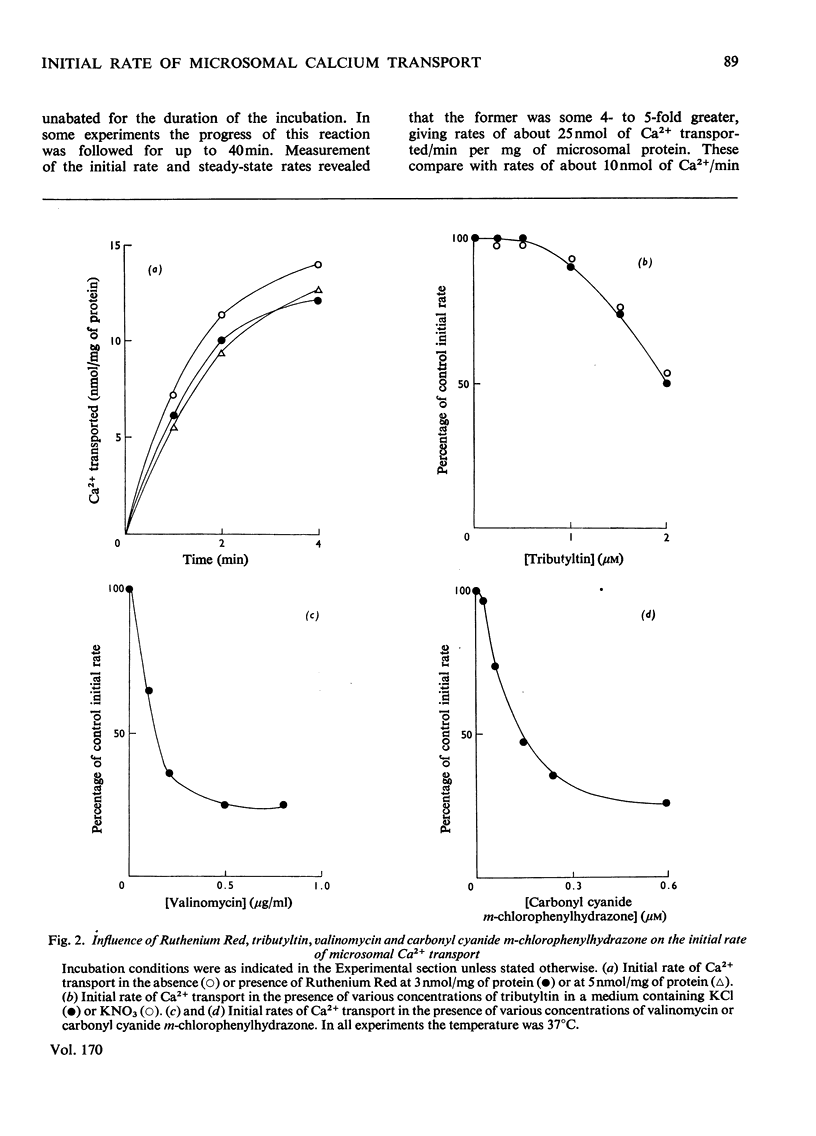
Measurements of the initial rate of Ca2+ transport by rat liver microsomal preparations reveal the existence of two phases of transport activity. The first, a phase of rapid transport, is complete by 3-5 min, at which time the second (slower) phase begins; this remains linear for up to at least 40 min. The initial phase is minimal in the absence of MgATP. The initial rate of Ca2+ transport reaches values as high as 25 nmol/min per mg of protein; the Km for Ca2+total is 1-2 micrometer and that for MgATPtotal about 500 micrometer. Ruthenium Red (3-5 nmol/mg of protein) has little effect on the initial rate of transport, whereas tributylin (2 micrometer) inhibits equally in a KC1- or a KNO3-containing medium. Compunds that collapse components of the proton electrochemical gradient in mitochondria (valinomycin and carbonyl cyanide m-chlorophenylhydrazone) each inhibit by 70-80% the initial rate of microsomal Ca2+ transport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Adenosine triphosphatase in the microsomal fraction from rat brain. Biochem J. 1962 Jun;83:527–533. doi: 10.1042/bj0830527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. The biochemistry of organotin compounds: trialkyltins and oxidative phosphorylation. Biochem J. 1958 Jul;69(3):367–376. doi: 10.1042/bj0690367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash G. R., Bygrave F. L. Ruthenium red as a probe in assessing the potential of mitochondria to control intracellular calcium in liver. FEBS Lett. 1977 Jun 15;78(2):166–168. doi: 10.1016/0014-5793(77)80297-4. [DOI] [PubMed] [Google Scholar]
- Bruns D. E., McDonald J. M., Jarett L. Energy-dependent calcium transport in endoplasmic reticulum of adipocytes. J Biol Chem. 1976 Nov 25;251(22):7191–7197. [PubMed] [Google Scholar]
- Farber J. L., El-Mofty S. K., Schanne F. A., Aleo J. J., Jr, Serroni A. Intracellular calcium homeostasis in galactosamine-intoxicated rat liver cells. Active sequestration of calcium by microsomes and mitochondria. Arch Biochem Biophys. 1977 Jan 30;178(2):617–624. doi: 10.1016/0003-9861(77)90233-8. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. F., Landon E. J., Debbas G., Hurwitz L. A calcium pump in vascular smooth muscle. Science. 1972 Apr 21;176(4032):305–306. doi: 10.1126/science.176.4032.305. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Estimation of membrane potential and pH difference across the cristae membrane of rat liver mitochondria. Eur J Biochem. 1969 Feb;7(4):471–484. doi: 10.1111/j.1432-1033.1969.tb19633.x. [DOI] [PubMed] [Google Scholar]
- Moore L., Chen T., Knapp H. R., Jr, Landon E. J. Energy-dependent calcium sequestration activity in rat liver microsomes. J Biol Chem. 1975 Jun 25;250(12):4562–4568. [PubMed] [Google Scholar]
- Moore L., Hurwitz L., Davenport G. R., Landon E. J. Energy-dependent calcium uptake activity of microsomes from the aorta of normal and hypertensive rats. Biochim Biophys Acta. 1975 Dec 16;413(3):432–443. doi: 10.1016/0005-2736(75)90126-1. [DOI] [PubMed] [Google Scholar]
- Moore L., Rodman Davenport G., Landon E. J. Calcium uptake of a rat liver microsomal subcellular fraction in response to in vivo administration of carbon tetrachloride. J Biol Chem. 1976 Feb 25;251(4):1197–1201. [PubMed] [Google Scholar]
- Reed K. C., Bygrave F. L. The inhibition of mitochondrial calcium transport by lanthanides and ruthenium red. Biochem J. 1974 May;140(2):143–155. doi: 10.1042/bj1400143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Watson E. L., Siegel I. A. Effects of autonomic agents and cyclic AMP on calcium accumulation and release in dog submandibular microsomes. Biochem Pharmacol. 1977 Jan 15;26(2):125–127. doi: 10.1016/0006-2952(77)90383-5. [DOI] [PubMed] [Google Scholar]


