Abstract
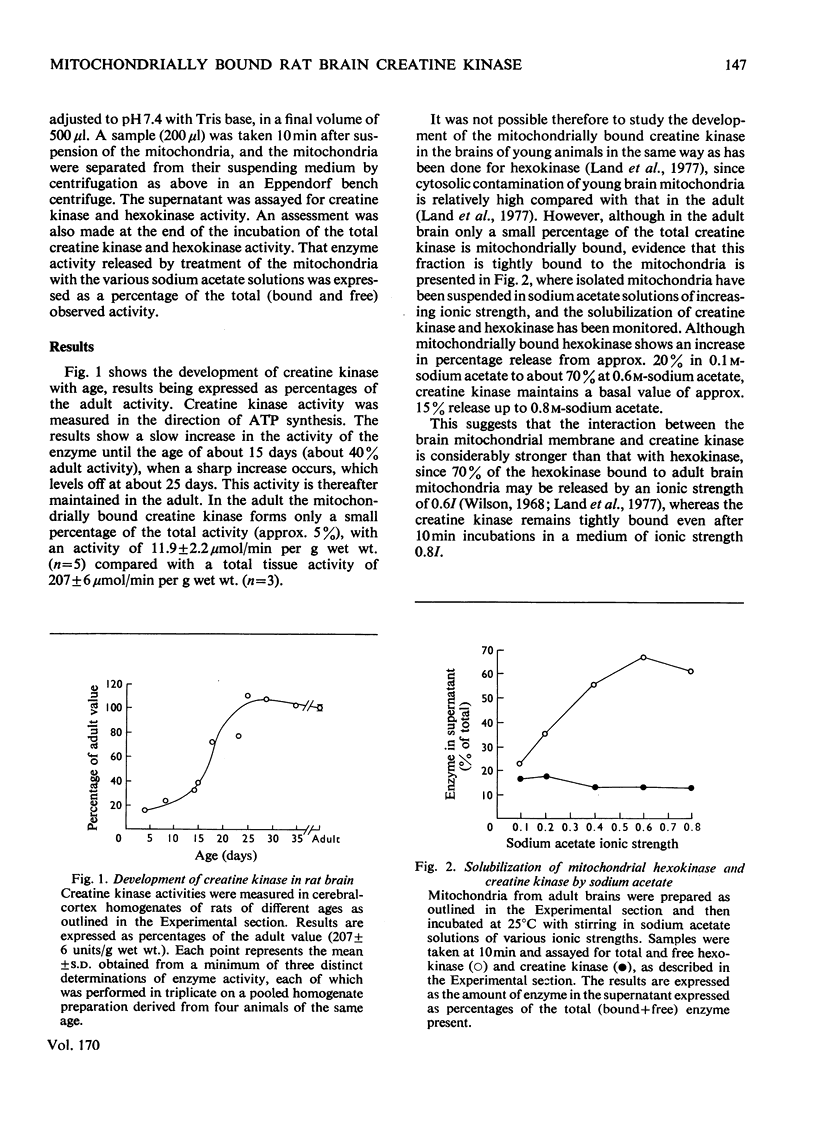
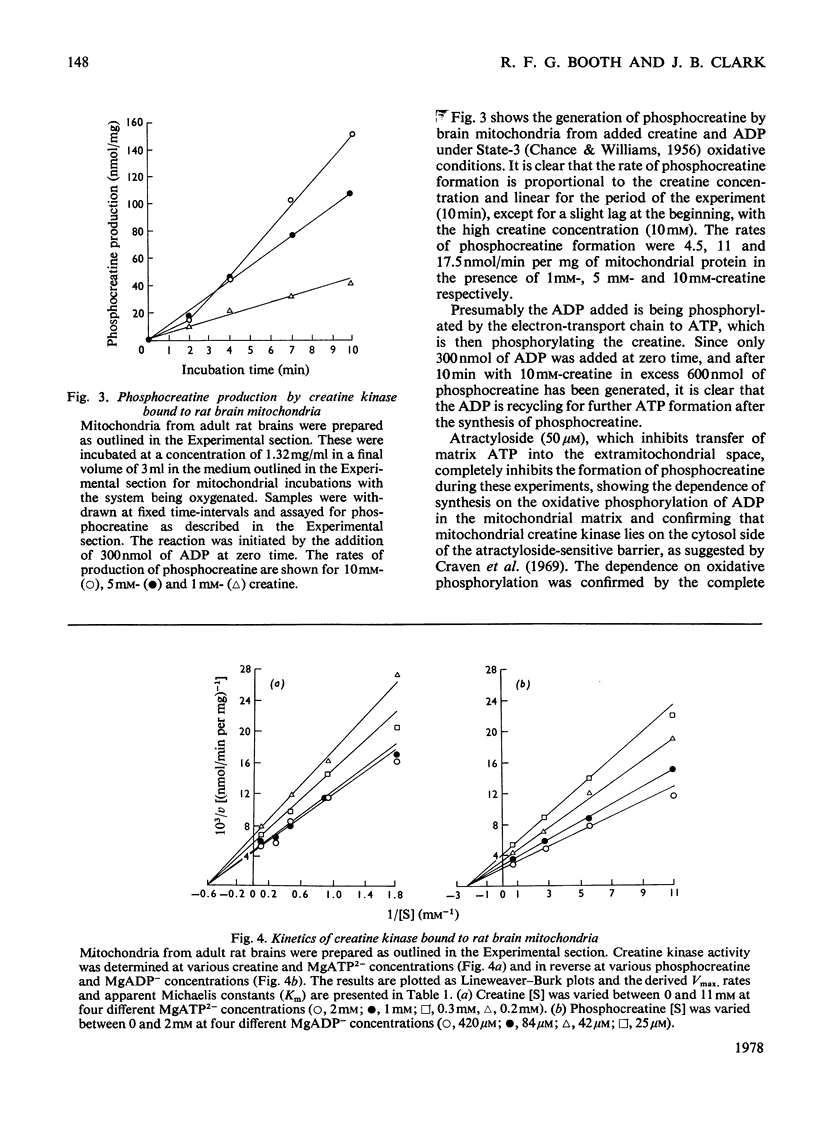
1. The development of the total rat brain creatine kinase was studied in brain homogenates. Until approx. 14–15 days after birth, the activity remains less than one-third that of the adult activity (207±6 units/g wet wt. s.d.; n=3). Over the next 10 days the activity increases markedly to the adult value and thereafter remains essentially constant. 2. In the adult brain, approx. 5% (11.9±2.2 units/g wet wt. s.d.; n=5) of the total creatine kinase is associated with the mitochondrial fraction. This creatine kinase could not be solubilized by sodium acetate solutions of up to 0.8m concentration, whereas 66% of the hexokinase associated with brain mitochondria was released under these conditions. 3. Rat brain mitochondria incubated in the presence of various concentrations of creatine (1, 5 and 10mm) and ADP (100μm) synthesized phosphocreatine at rates of approx. 4.5, 11 and 17.5nmol/min per mg of mitochondrial protein. Atractyloside (50μm) or oligomycin (1.5μg/mg of mitochondrial protein) completely inhibited the synthesis of phosphocreatine. 4. The apparent Km and Vmax. values of the mitochondrially bound rat brain creatine kinase were determined in both directions. The Vmax. in the direction of phosphocreatine synthesis is 237nmol/min per mg of mitochondrial protein, with an apparent Km for creatine of 1.67mm and for MgATP2− of 0.1mm, and in the reverse direction Vmax. is 489nmol/min per mg of mitochondrial protein, with an apparent Km for phosphocreatine of 0.4mm and for MgADP− of 27μm. 5. The results are discussed with reference to the role that the mitochondrially bound creatine kinase may play in the development of brain energy metabolism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Effect of pH and metal ion concentration on the equilibrium hydrolysis of adenosine triphosphate to adenosine diphosphate. J Biol Chem. 1968 Apr 10;243(7):1337–1343. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Clark J. B., Nicklas W. J. The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem. 1970 Sep 25;245(18):4724–4731. [PubMed] [Google Scholar]
- Craven P. A., Goldblatt P. J., Basford R. E. Brain hexokinase. The preparation of inner and outer mitochondrial membranes. Biochemistry. 1969 Sep;8(9):3525–3532. doi: 10.1021/bi00837a007. [DOI] [PubMed] [Google Scholar]
- Hernandez A., Crane R. K. Association of heart hexokinase with subcellular structure. Arch Biochem Biophys. 1966 Jan;113(1):223–229. doi: 10.1016/0003-9861(66)90176-7. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Heldt H. W., Klingenberg M. High activity of creatine kinase in mitochondria from muscle and brain and evidence for a separate mitochondrial isoenzyme of creatine kinase. Biochem Biophys Res Commun. 1964 Aug 11;16(6):516–521. doi: 10.1016/0006-291x(64)90185-8. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Lehninger A. L. Creatine kinase of rat heart mitochondria. Coupling of creatine phosphorylation to electron transport. J Biol Chem. 1973 Jul 10;248(13):4803–4810. [PubMed] [Google Scholar]
- Krzanowski J., Matschinsky F. M. Regulation of phosphofructokinase by phosphocreatine and phosphorylated glycolytic intermediates. Biochem Biophys Res Commun. 1969 Mar 31;34(6):816–823. doi: 10.1016/0006-291x(69)90253-8. [DOI] [PubMed] [Google Scholar]
- Land J. M., Booth R. F., Berger R., Clark J. B. Development of mitochondrial energy metabolism in rat brain. Biochem J. 1977 May 15;164(2):339–348. doi: 10.1042/bj1640339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapin E. P., Maker H. S., Lehrer G. M. Changes during development of mouse brain in the activities and subcellular distributions of creatine and adenylate kinases. J Neurochem. 1974 Sep;23(3):465–469. doi: 10.1111/j.1471-4159.1974.tb06047.x. [DOI] [PubMed] [Google Scholar]
- Moore C. L., Jöbsis F. F. Some studies on the control of respiration in rat brain mitochondrial preparations. Arch Biochem Biophys. 1970 May;138(1):295–305. doi: 10.1016/0003-9861(70)90310-3. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Chernousova G. B., Gukovsky D. E., Smirnov V. N., Chazov E. I. Studies of energy transport in heart cells. Mitochondrial isoenzyme of creatine phosphokinase: kinetic properties and regulatory action of Mg2+ ions. Eur J Biochem. 1975 Sep 1;57(1):273–290. doi: 10.1111/j.1432-1033.1975.tb02299.x. [DOI] [PubMed] [Google Scholar]
- Saks V. A., Chernousova G. B., Vetter R., Smirnov V. N., Chazov E. I. Kinetic properties and the functional role of particulate MM-isoenzyme of creatine phosphokinase bound to heart muscle myofibrils. FEBS Lett. 1976 Mar 1;62(3):293–296. doi: 10.1016/0014-5793(76)80078-6. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Harris R. L., Veloso D., Veech E. H. Freeze-blowing: a new technique for the study of brain in vivo. J Neurochem. 1973 Jan;20(1):183–188. doi: 10.1111/j.1471-4159.1973.tb12115.x. [DOI] [PubMed] [Google Scholar]
- Vial C., Godinot C., Gautheron D. Membranes: creatine kinase (E.C.2.7.3.2.) in pig heart mitochondria. Properties and role in phosphate potential regulation. Biochimie. 1972;54(7):843–852. doi: 10.1016/s0300-9084(72)80005-1. [DOI] [PubMed] [Google Scholar]
- WOOD T., SWANSON P. D. THE OCCURRENCE OF ADENOSINE TRIPHOSPHATE-CREATINE PHOSPHOTRANSFERASE IN PARTICULATE FRACTIONS FROM CEREBRAL TISSUE AND ITS COUPLING TO THE SODIUM-STIMULATED MICROSOMAL ADENOSINE-TRIPHOSPHATASE. J Neurochem. 1964 Apr;11:301–307. doi: 10.1111/j.1471-4159.1964.tb06144.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Dutton P. L. Thermodynamic relationships in mitochondrial oxidative phosphorylation. Annu Rev Biophys Bioeng. 1974;3(0):203–230. doi: 10.1146/annurev.bb.03.060174.001223. [DOI] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem. 1968 Jul 10;243(13):3640–3647. [PubMed] [Google Scholar]


