Abstract
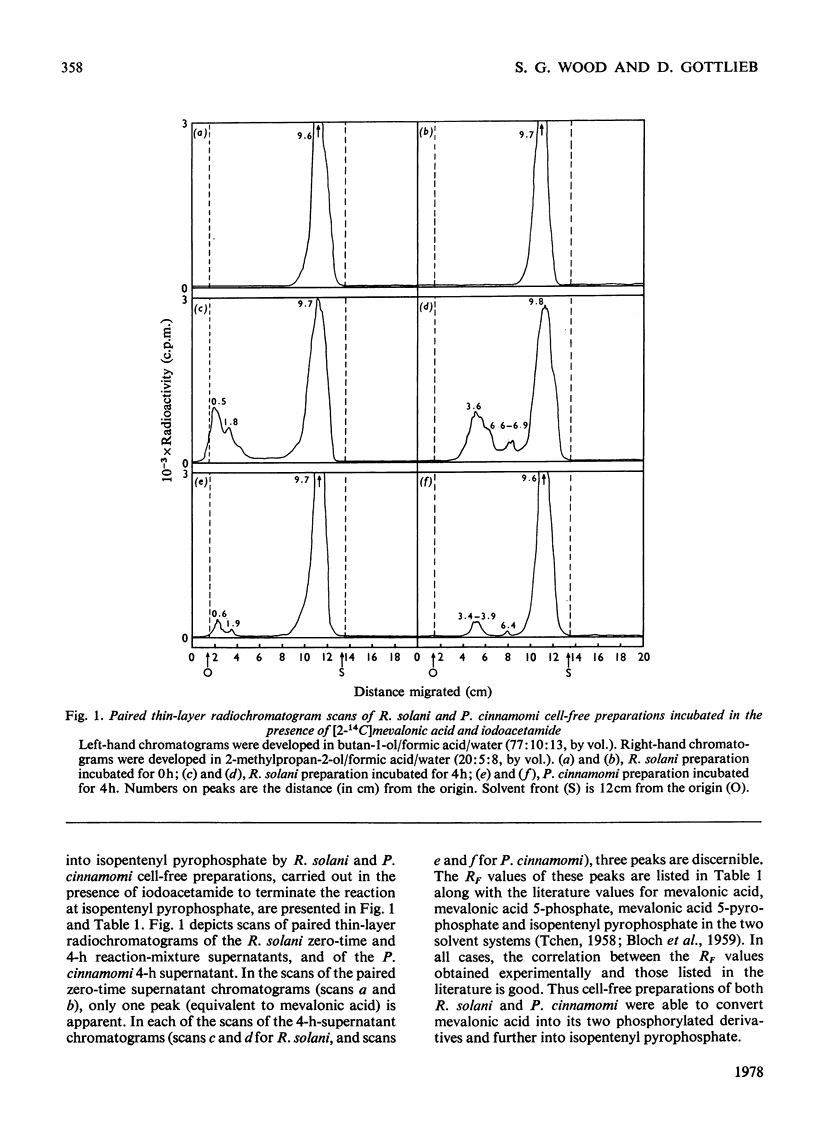
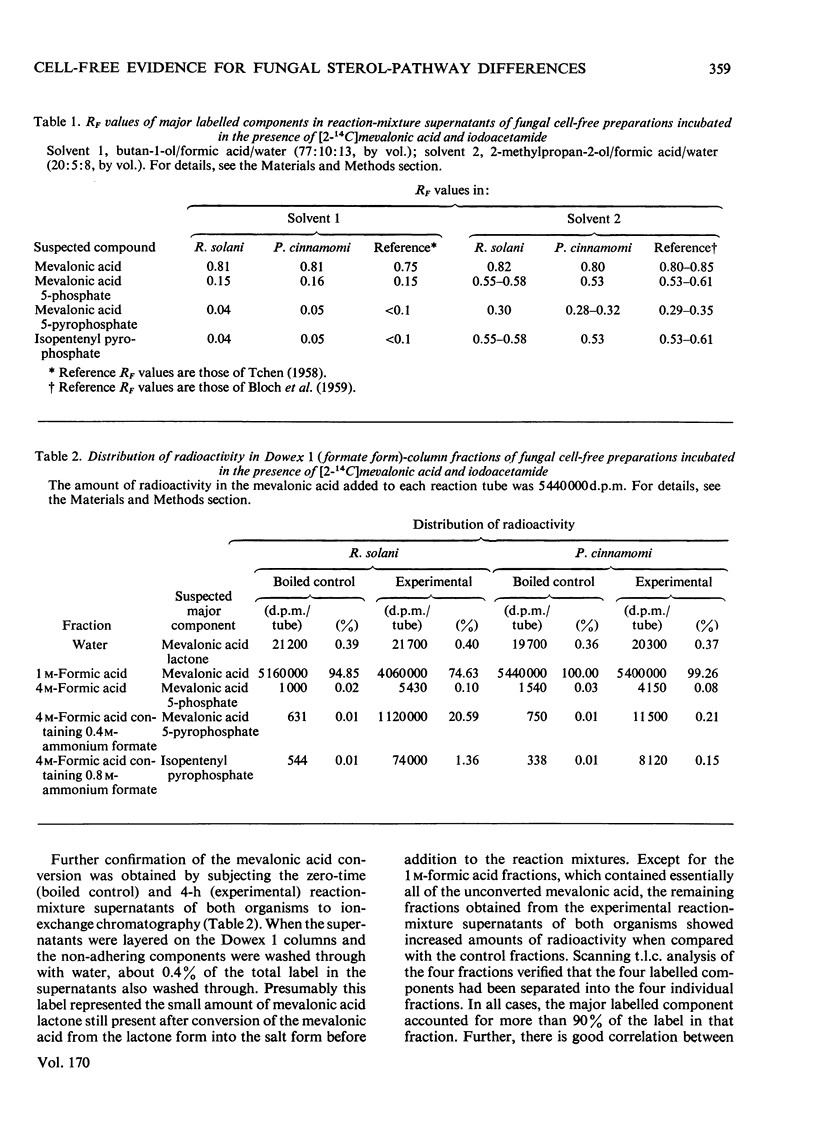
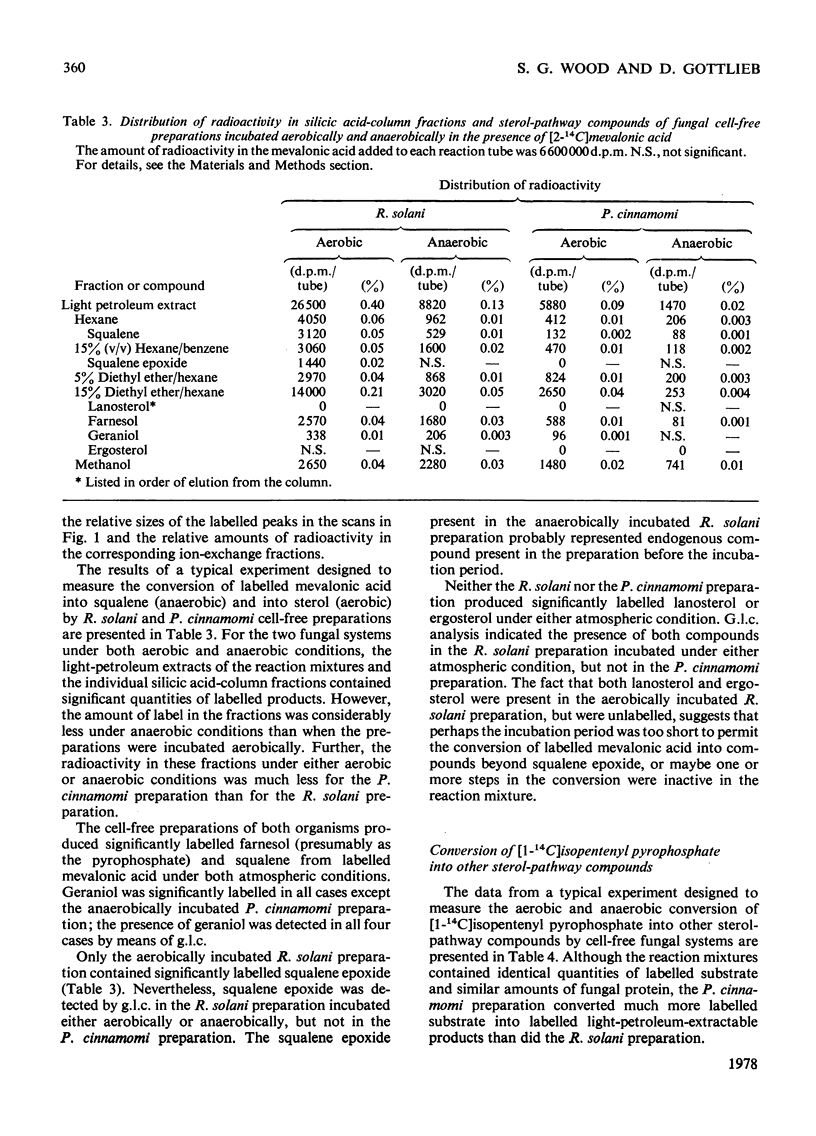
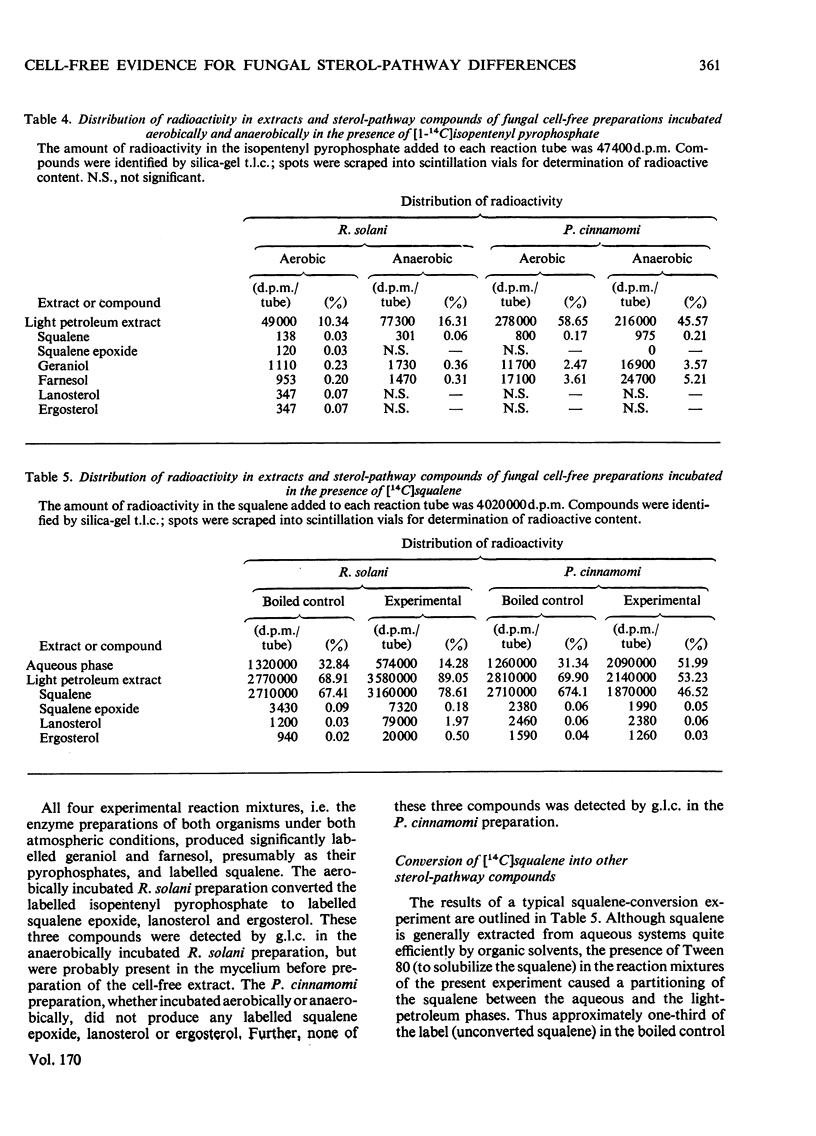
Cell-free preparations of both Rhizoctonia solani, a sterol-synthesizing fungus, and Phytophthora cinnamomi, a non-sterol-synthesizing fungus, incubated in the presence of [2(-14)C]mevalonate and iodacetamide, converted the mevalonate into labelled mevalonate 5-phosphate, mevalonate 5-pyrophosphate and isopentenyl pyrophosphate. In the absence of iodoacetamide, but under anaerobic conditions, the same preparations converted the mevalonate into labelled geraniol, farnesol and squalene, the first two compounds presumably as their pyrophosphates. When cell-free preparations of both organisms were incubated aerobically in the presence of [1(-14)C]isopentenyl pyrophosphate, only labelled geraniol, farnesol and squalene were recovered from the P. cinnamomi reaction mixture, whereas labelled geraniol, farnesol, squalene, squalene epoxide, lanosterol and ergosterol were present in the R. solani reaction mixture. When these same preparations were incubated in the presence of 14C-labelled squalene, labelled squalene epoxide, lanosterol and ergosterol were recovered from the R. solani reaction mixture. In contrast, the P. cinnamomi preparation was unable to convert the squalene into products further along the sterol pathway; instead, a portion of the labelled squalene was converted into water-soluble products, indicating the possible existence of a squalene-degradation process in this organism. It appears that the block in the sterol biosynthetic pathway of P. cinnamomi occurs at the level of squalene epoxidation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOCH K., CHAYKIN S., PHILLIPS A. H., DE WAARD A. Mevalonic acid pyrophosphate and isopentenylpyrophosphate. J Biol Chem. 1959 Oct;234:2595–2604. [PubMed] [Google Scholar]
- Kawaguchi A., Nozoe S., Okuda S. Subcellular distribution of sesterterpene- and sterol-biosynthesizing activities in Cochliobolus heterostrophus. Biochim Biophys Acta. 1973 Mar 8;296(3):615–623. [PubMed] [Google Scholar]
- Popják G., Edmond J., Clifford K., Williams V. Biosynthesis and structure of a new intermediate between farnesyl pyrophosphate and squalene. J Biol Chem. 1969 Apr 10;244(7):1897–1918. [PubMed] [Google Scholar]
- Shechter I., Sweat F. W., Bloch K. Comparative properties of 2,3-oxidosqualene-lanosterol cyclase from yeast and liver. Biochim Biophys Acta. 1970 Dec 16;220(3):463–468. doi: 10.1016/0005-2744(70)90277-9. [DOI] [PubMed] [Google Scholar]
- TCHEN T. T. Mevalonic kinase: purification and properties. J Biol Chem. 1958 Nov;233(5):1100–1103. [PubMed] [Google Scholar]
- Wood S. G., Gottlieb D. Evidence from mycelial studies for differences in the sterol biosynthetic pathway of Rhizoctonia solani and Phytophthora cinnamomi. Biochem J. 1978 Feb 15;170(2):343–354. doi: 10.1042/bj1700343. [DOI] [PMC free article] [PubMed] [Google Scholar]


