Abstract
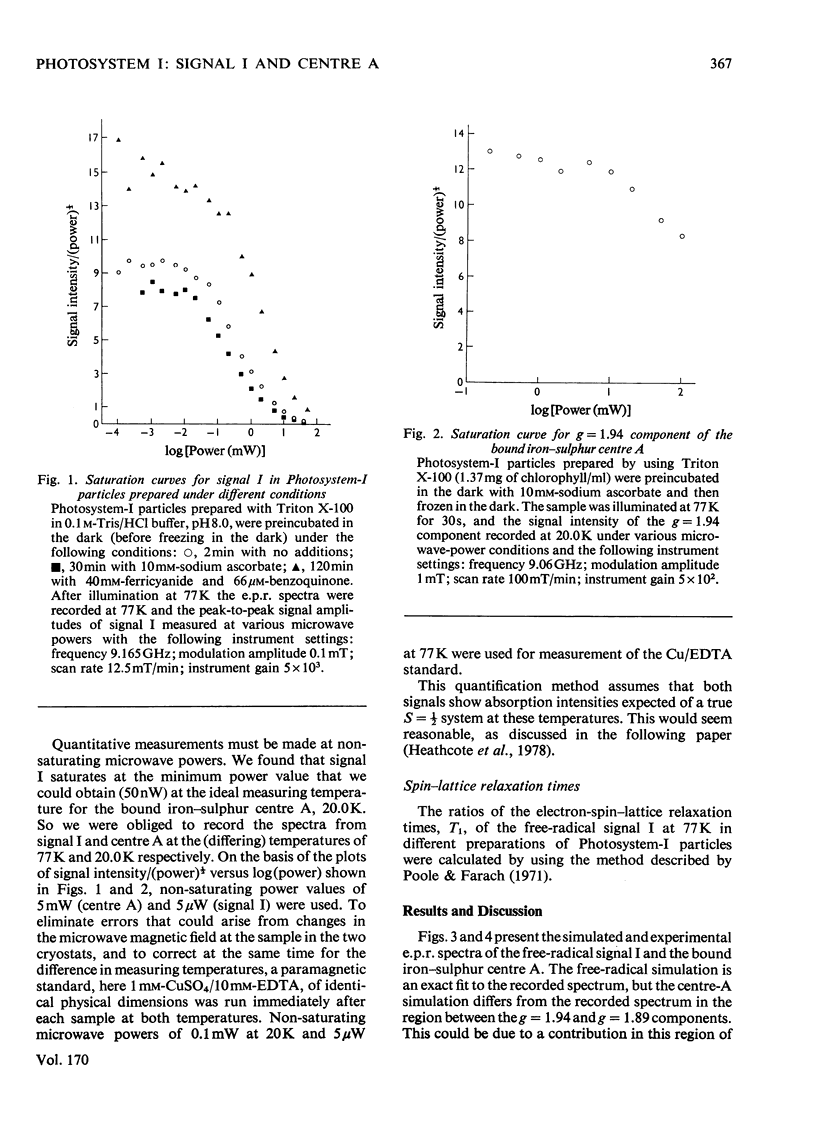
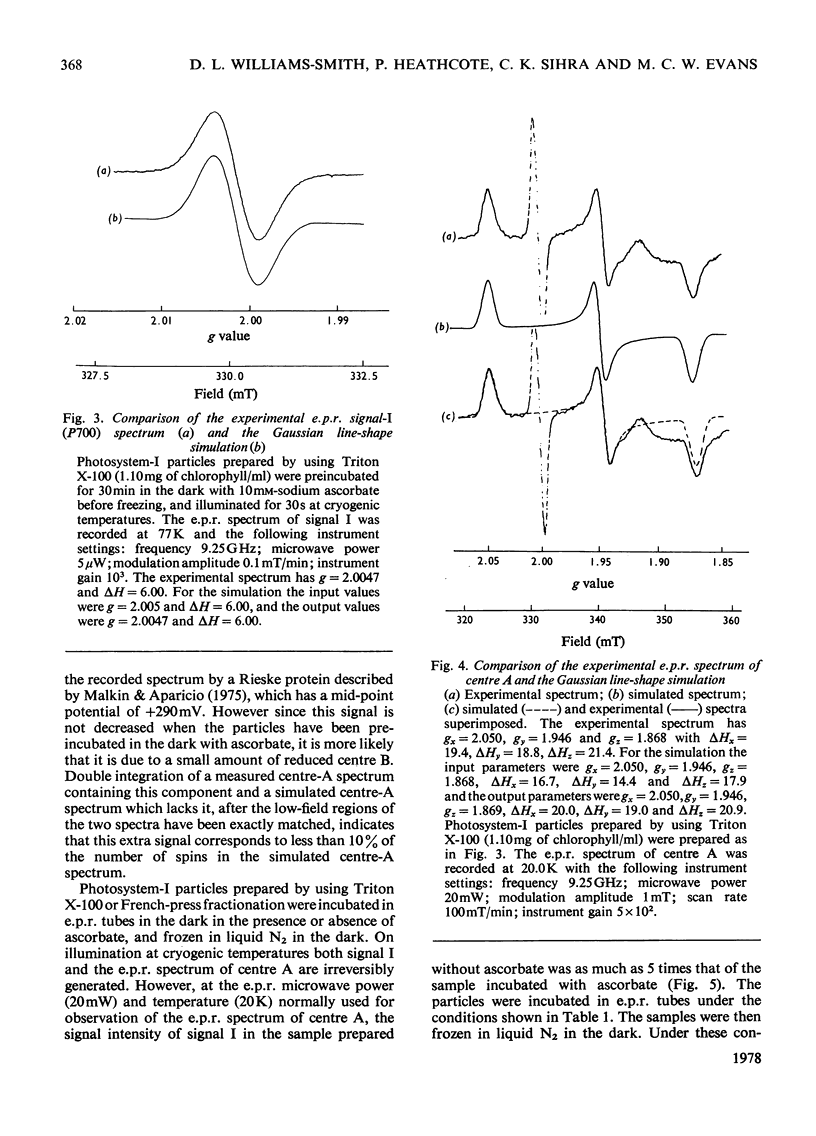
E.p.r. spectrometry was used to investigate the quantitative relationships between the oxidized chlorophyll free-radical signal I and the reduced iron-sulphur centre-A signal generated on illuminating Photosystem-I particles at cryogenic temperatures. In Photosystem-I particles prepared by using the French press or Triton X-100, at pH8.0 in the presence and absence of ascorbate and at pH 10.0 in the presence of ascorbate, the size of the light-induced signal I and iron-sulphur centre-A signals, corresponded to equal numbers of unpaired electron spins in each component. At 77K the spin-lattice relaxation time, T1, of the free radical signal I in samples of Photosystem-I particles prepared with Triton X-100 in the absence of ascorbate was 0.68 times the T1 value in the presence of ascorbate. Such changes in relaxation time can account for the different quantitative conclusions incorrectly arrived at from measurements made at saturating microwave powers [Bearden & Malkin (1976) Biochem. Biophys. Acta 430, 538-547; Malkin & Bearden (1976) FEBS Lett. 69, 216-220]. In the presence of benzoquinone and ferricyanide the ratio of free radical to centre A was 2.96:1, and at 77K the T1 was 0.50 times the T1 for ascorbate-treated samples. Here free radicals from bulk chlorophyll are generated in addition to those from the reaction-centre chlorophyll.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEINERT H., KOK B. AN ATTEMPT AT QUANTITATION OF THE SHARP LIGHT-INDUCED ELECTRON PARAMAGNETIC RESONANCE SIGNAL IN PHOTOSYNTHETIC MATERIALS. Biochim Biophys Acta. 1964 Sep 25;88:278–288. doi: 10.1016/0926-6577(64)90183-4. [DOI] [PubMed] [Google Scholar]
- BEINERT H., KOK B., HOCH G. The light induced electron paramagnetic resonance signal of photocatalyst P700. Biochem Biophys Res Commun. 1962 Apr 20;7:209–212. doi: 10.1016/0006-291x(62)90176-6. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Correlation of reaction-center chlorophyll (P-700) oxidation and bound iron-sulfur protein photoreduction in chloroplast photosystem I at low temperatures. Biochim Biophys Acta. 1976 Jun 8;430(3):538–547. doi: 10.1016/0005-2728(76)90029-3. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Quantitative EPR studies of the primary reaction of photosystem I in chloroplasts. Biochim Biophys Acta. 1972 Dec 14;283(3):456–468. doi: 10.1016/0005-2728(72)90262-9. [DOI] [PubMed] [Google Scholar]
- Commoner B., Heise J. J., Townsend J. LIGHT-INDUCED PARAMAGNETISM IN CHLOROPLASTS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):710–718. doi: 10.1073/pnas.42.10.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Cammack R. The effect of the redox state of the bound iron-sulphur centres in spinach chloroplasts on the reversibility of P700 photooxidation at low temperatures. Biochem Biophys Res Commun. 1975 Mar 3;63(1):187–193. doi: 10.1016/s0006-291x(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Cammack R. The properties of the primary electron acceptor in the Photosystem I reaction centre of spinach chloroplasts and its interaction with P700 and the bound ferredoxin in various oxidation-reduction states. Biochem J. 1976 Jul 15;158(1):71–77. doi: 10.1042/bj1580071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Slabas A. R. The oxidation-reduction potential of the reaction-centre chlorophyll (P700) in Photosystem I. Evidence for multiple components in electron-paramagnetic-resonance signal 1 at low temperature. Biochem J. 1977 Jan 15;162(1):75–85. doi: 10.1042/bj1620075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Heathcote P., Williams-Smith D. L., Evans M. C. Quantitative electron-paramagnetic-resonance measurements of the electron-transfer components of the photosystem-I reaction centre. The reaction-centre chlorophyll (P700), the primary electron acceptor X and bound iron-sulphur centre A. Biochem J. 1978 Feb 15;170(2):373–378. doi: 10.1042/bj1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOK B. On the reversible absorption change at 705 mu in photosynthetic organisms. Biochim Biophys Acta. 1956 Nov;22(2):399–401. doi: 10.1016/0006-3002(56)90172-x. [DOI] [PubMed] [Google Scholar]
- Malkin R., Aparicio P. J. Identification of a g equals 1.90 high-potential iron-sulfur protein in chloroplasts. Biochem Biophys Res Commun. 1975 Apr 21;63(4):1157–1160. doi: 10.1016/0006-291x(75)90690-7. [DOI] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. The effect of alkaline pH on chloroplasm photosystem I reactions at cryogenic temperature. FEBS Lett. 1976 Oct 15;69(1):216–220. doi: 10.1016/0014-5793(76)80690-4. [DOI] [PubMed] [Google Scholar]
- McIntosh A. R., Chu M., Bolton J. R. Flash photolysis electron spin resonance studies of the electron acceptor species at low temperatures in photosystem I of spinach subchloroplast particles. Biochim Biophys Acta. 1975 Feb 17;376(2):308–314. doi: 10.1016/0005-2728(75)90023-7. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Warden J. T., Jr, Bolton J. R. Simultaneous quantitative comparison of the optical changes at 700 nm (p700) and electron spin resonance signals in system I of green plant photosynthesis. J Am Chem Soc. 1973 Sep 19;95(19):6435–6436. doi: 10.1021/ja00800a046. [DOI] [PubMed] [Google Scholar]


