Abstract
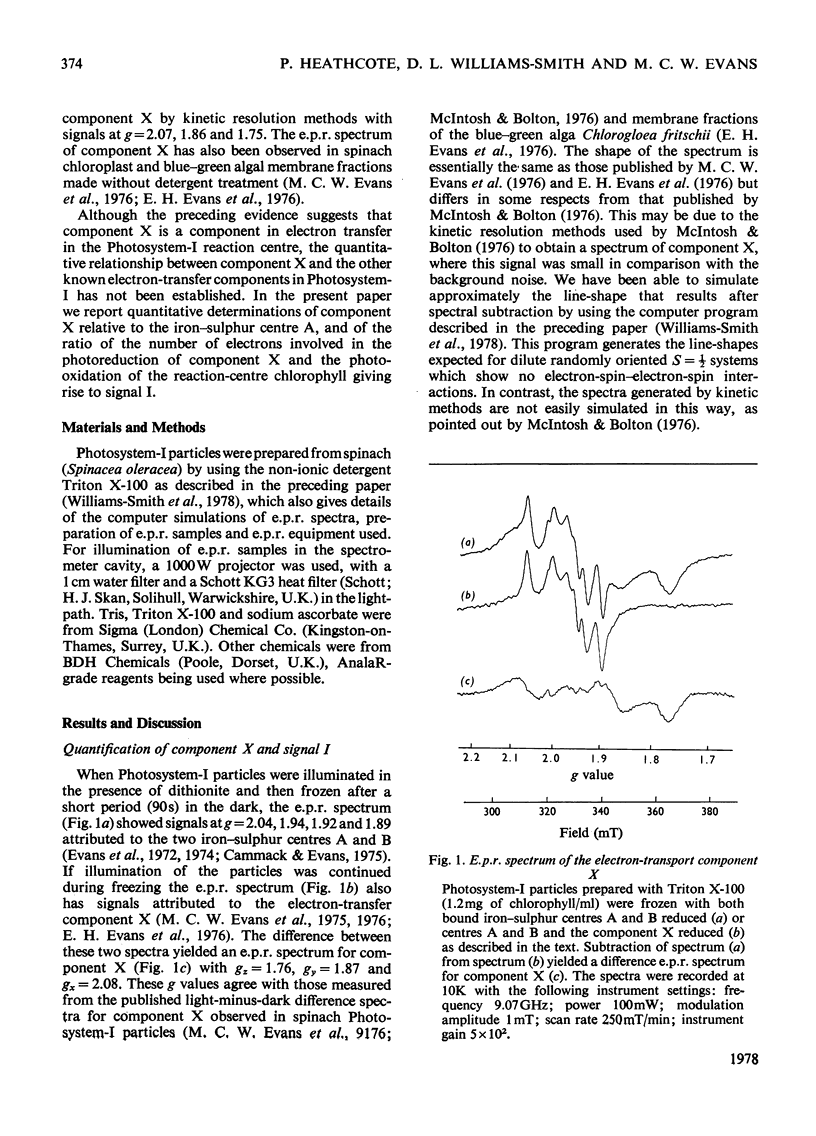
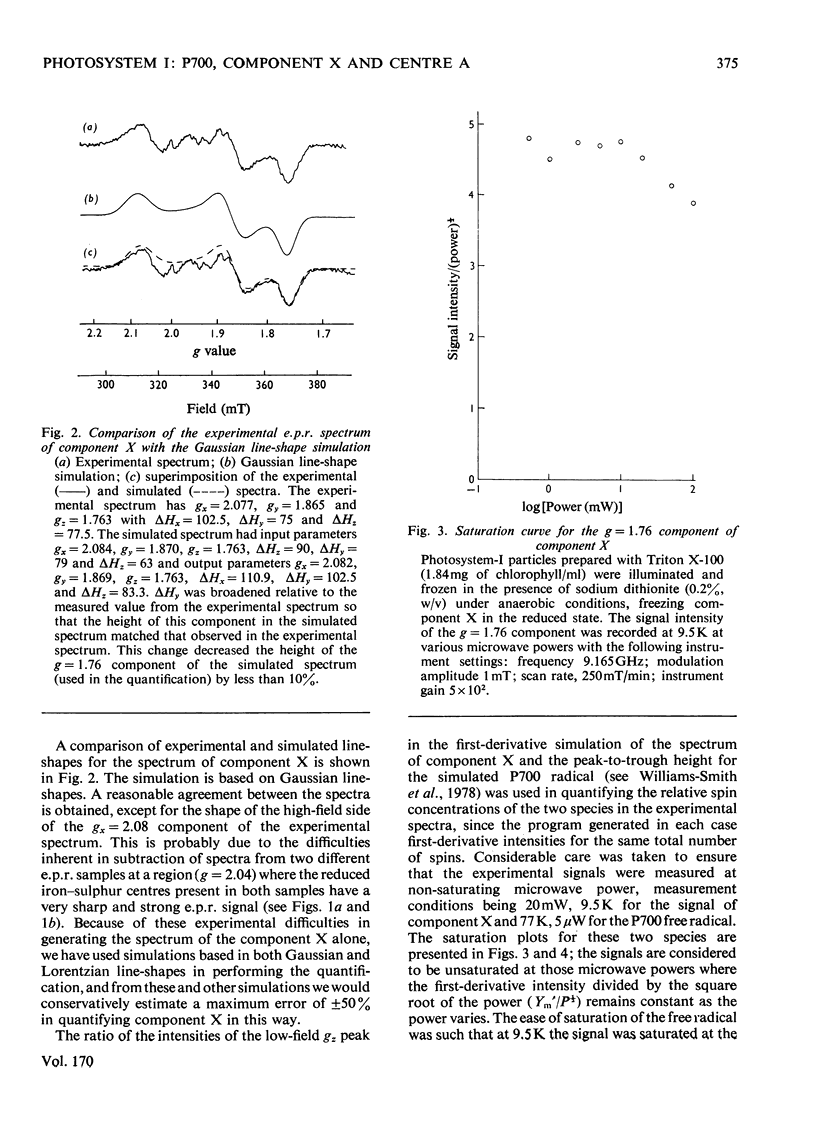
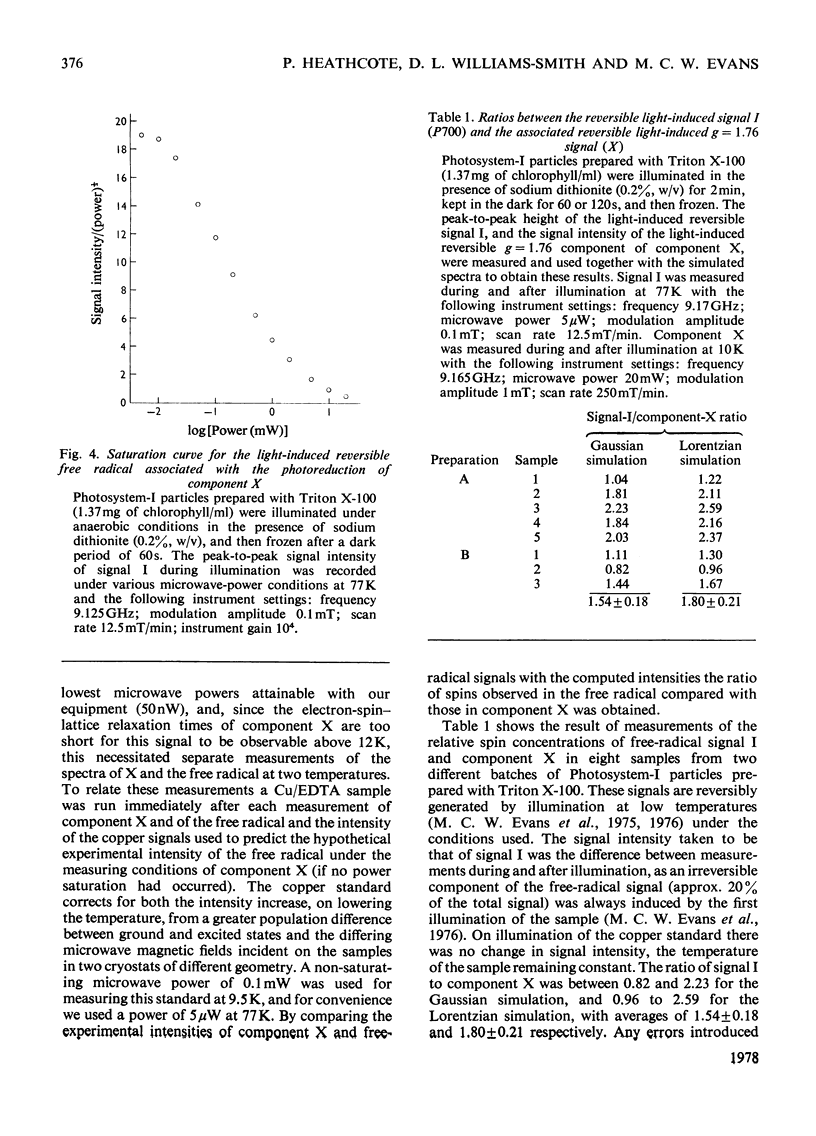
An e.p.r. spectrum of the reduced form of the electron-transport component (X), thought to be the primary electron acceptor of Photosystem I, was obtained. By using line-shape simulations of this component and the free-radical e.p.r. signal I of the oxidized reaction-centre chlorophyll (P700), it was possible to determine the ratio of the number of electron spins to which these signals correspond in Photosystem-I particles under a variety of conditions. On illumination at cryogenic temperatures of Photosystem-I preparations, in which both bound iron-sulphur centres A and B were reduced, the measured ratio of free radical to component X varied between 1.04 and 2.23, with an average value of 1.54 +/- 0.18 where a Gaussian line-shape is assumed for the component-X signal in the simulation. The error in this measurement is estimated to be up to 50%. In a similar way component X and centre A of the bound iron-sulphur protein were quantified, the ratio between these two components varying between 1.26 and 0.61 with an average value of 0.75 +/- 0.06. These results indicate that the quantitative relationship, in terms of net electron spins, between centre A, component X and P700 is of the order to be expected if component X is indeed the primary electron acceptor in Photosystem I and a component of the photosynthetic electron-transport chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H., KOK B., HOCH G. The light induced electron paramagnetic resonance signal of photocatalyst P700. Biochem Biophys Res Commun. 1962 Apr 20;7:209–212. doi: 10.1016/0006-291x(62)90176-6. [DOI] [PubMed] [Google Scholar]
- Bearden A. J., Malkin R. Quantitative EPR studies of the primary reaction of photosystem I in chloroplasts. Biochim Biophys Acta. 1972 Dec 14;283(3):456–468. doi: 10.1016/0005-2728(72)90262-9. [DOI] [PubMed] [Google Scholar]
- Cammack R., Evans M. C. E.P.R. spectra of iron-sulphur proteins in dimethylsulphoxide solutions: evidence that chloroplast photosystem I particles contain 4Fe-4S centres. Biochem Biophys Res Commun. 1975 Nov 17;67(2):544–549. doi: 10.1016/0006-291x(75)90846-3. [DOI] [PubMed] [Google Scholar]
- Commoner B., Heise J. J., Townsend J. LIGHT-INDUCED PARAMAGNETISM IN CHLOROPLASTS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):710–718. doi: 10.1073/pnas.42.10.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. H., Cammack R. Properties of the primary electron acceptor complex of photosystem I in the blue green alga Chlorogloea fritschii. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1212–1218. doi: 10.1016/0006-291x(76)90326-0. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Cammack R. The effect of the redox state of the bound iron-sulphur centres in spinach chloroplasts on the reversibility of P700 photooxidation at low temperatures. Biochem Biophys Res Commun. 1975 Mar 3;63(1):187–193. doi: 10.1016/s0006-291x(75)80028-3. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Reeves S. G., Cammack R. Determination of the oxidation-reduction potential of the bound iron-sulphur proteins of the primary electron acceptor complex of photosystem I in spinach chloroplasts. FEBS Lett. 1974 Dec 1;49(1):111–114. doi: 10.1016/0014-5793(74)80644-7. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Sihra C. K., Cammack R. The properties of the primary electron acceptor in the Photosystem I reaction centre of spinach chloroplasts and its interaction with P700 and the bound ferredoxin in various oxidation-reduction states. Biochem J. 1976 Jul 15;158(1):71–77. doi: 10.1042/bj1580071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Lord A. V. Evidence for the role of a bound ferredoxin as the primary electron acceptor of photosystem I in spinach chloroplasts. Biochim Biophys Acta. 1972 Jun 23;267(3):530–537. doi: 10.1016/0005-2728(72)90181-8. [DOI] [PubMed] [Google Scholar]
- Heathcote P., Williams-Smith D. L., Evans M. C. Quantitative electron-paramagnetic-resonance measurements of the electron-transfer components of the photosystem-I reaction centre. The reaction-centre chlorophyll (P700), the primary electron acceptor X and bound iron-sulphur centre A. Biochem J. 1978 Feb 15;170(2):373–378. doi: 10.1042/bj1700373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOK B. On the reversible absorption change at 705 mu in photosynthetic organisms. Biochim Biophys Acta. 1956 Nov;22(2):399–401. doi: 10.1016/0006-3002(56)90172-x. [DOI] [PubMed] [Google Scholar]
- Kimura T., Tasaki A., Watari H. Studies on adrenal steroid hydroxylases. The magnetic susceptibility of oxidized and reduced adrenal iron-sulfur protein (adrenodoxin). J Biol Chem. 1970 Sep 10;245(17):4450–4452. [PubMed] [Google Scholar]
- Malkin R., Bearden A. J. Primary reactions of photosynthesis: photoreduction of a bound chloroplast ferredoxin at low temperature as detected by EPR spectroscopy. Proc Natl Acad Sci U S A. 1971 Jan;68(1):16–19. doi: 10.1073/pnas.68.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh A. R., Chu M., Bolton J. R. Flash photolysis electron spin resonance studies of the electron acceptor species at low temperatures in photosystem I of spinach subchloroplast particles. Biochim Biophys Acta. 1975 Feb 17;376(2):308–314. doi: 10.1016/0005-2728(75)90023-7. [DOI] [PubMed] [Google Scholar]
- Mcintosh A. R., Bolton J. R. Electron spin resonance spectrum of species "X" which may function as the primary electron acceptor in photosystem I of green plant photosynthesis. Biochim Biophys Acta. 1976 Jun 8;430(3):555–559. [PubMed] [Google Scholar]
- Moss T. H., Petering D., Palmer G. The magnetic susceptibility of oxidized and reduced ferredoxins from spinach and parsley and the high potential protein from Chromatium. J Biol Chem. 1969 May 10;244(9):2275–2277. [PubMed] [Google Scholar]


