Abstract
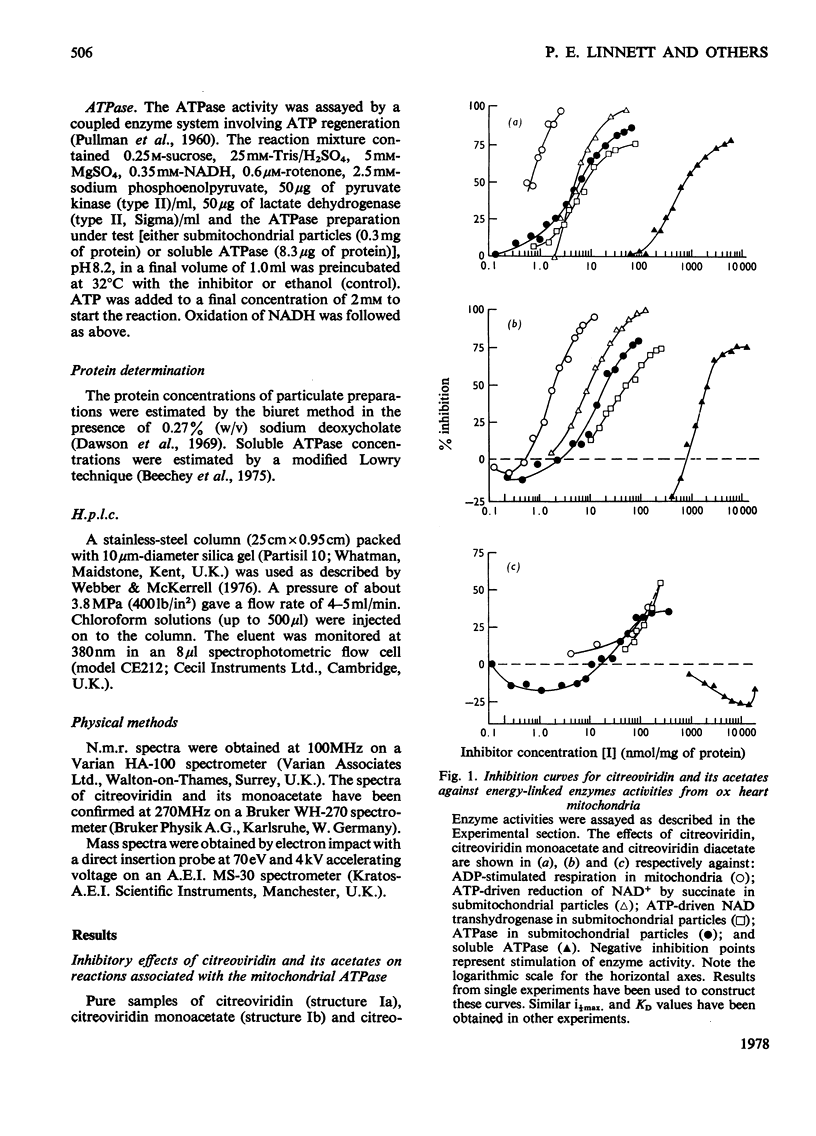
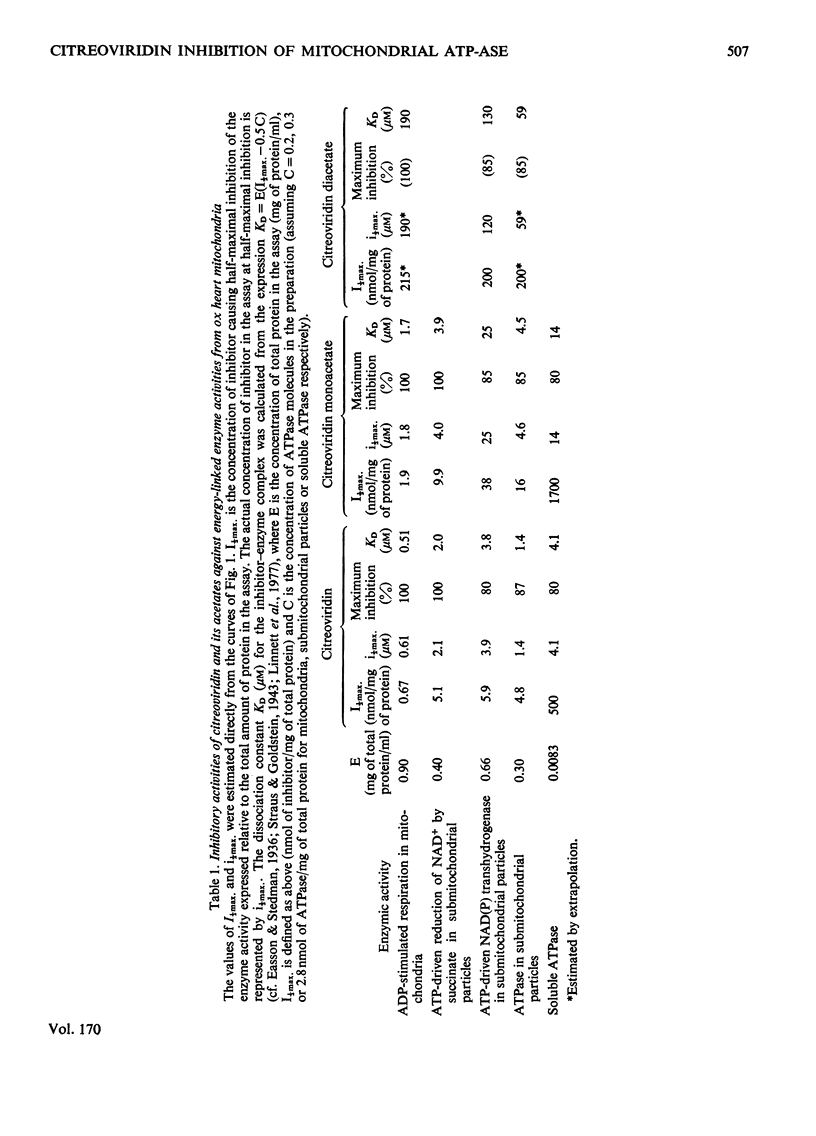
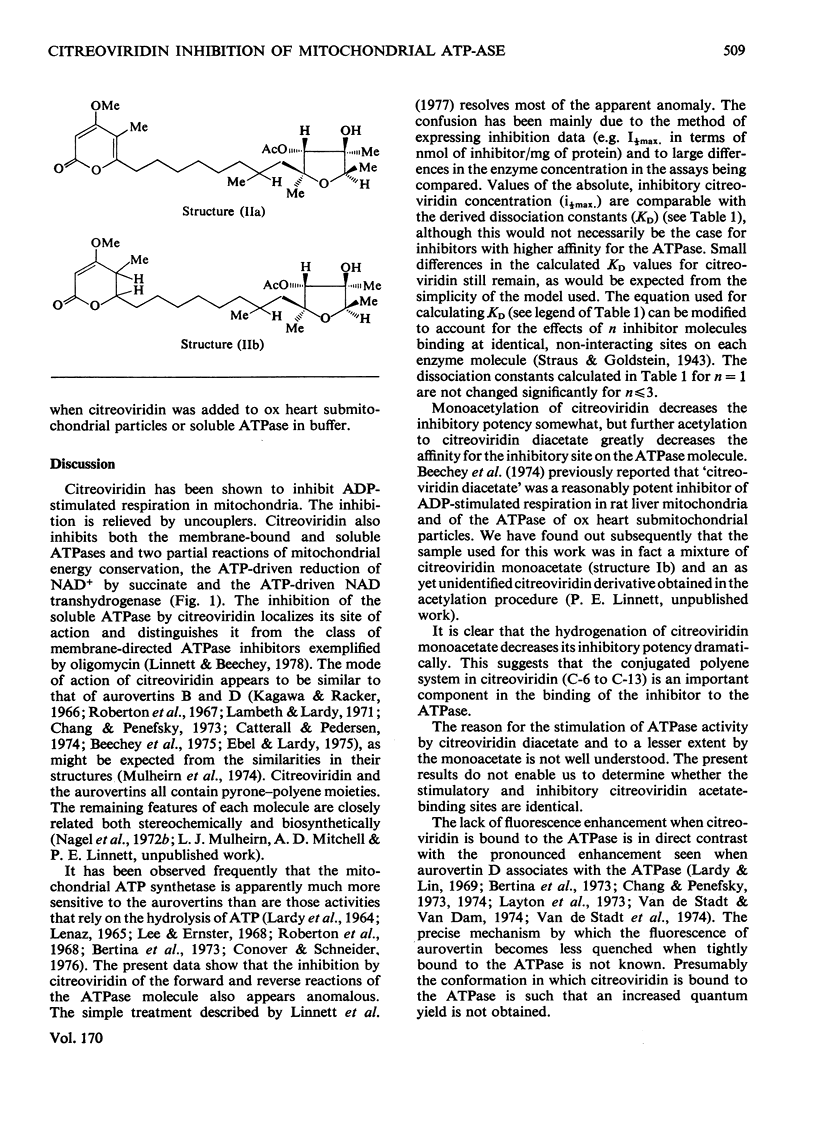
1. Citreoviridin was a potent inhibitor of the soluble mitochondrial ATPase (adenosine triphosphatase) similar to the closely related aurovertins B and D. 2. Citreoviridin inhibited the following mitochondrial energy-linked reactions also: ADP-stimulated respiration in whole mitochondria from ox heart and rat liver; ATP-driven reduction of NAD+ by succinate; ATP-driven NAD transhydrogenase and ATPase from ox heart submitochondrial particles. 3. The dissociation constant (KD) calculated by a simple law-of-mass-action treatment for the citreoviridin--ATPase complex was 0.5--4.2micron for ox-heart mitochondrial preparations and 0.15micron for rat liver mitochondria. 4. Monoacetylation of citreoviridin decreased its inhibitory potency (KD=2--25micron, ox heart; KD=0.7micron, rat liver). Diacetylation greatly decreased the inhibitory potency (KD=60--215micron, ox heart). 5. Hydrogenation of citreoviridin monoacetate diminished its inhibitory potency considerably. 6. No significant enhancement of fluorescence was observed when citreoviridin interacted with the mitochondrial ATPase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beechey R. B., Hubbard S. A., Linnett P. E., Mitchell A. D., Munn E. A. A simple and rapid method for the preparation of adenosine triphosphatase from submitochondrial particles. Biochem J. 1975 Jun;148(3):533–537. doi: 10.1042/bj1480533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertina R. M., Schrier P. I., Slater E. C. The binding of aurovertin to mitochondria, and its effect on mitochondrial respiration. Biochim Biophys Acta. 1973 Jun 28;305(3):503–518. doi: 10.1016/0005-2728(73)90072-8. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Chang T. M., Penefsky H. S. Energy-dependent enhancement of aurovertin fluorescence. An indicator of conformational changes in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1090–1098. [PubMed] [Google Scholar]
- Chang T., Penefsky H. S. Aurovertin, a fluorescent probe of conformational change in beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1973 Apr 25;248(8):2746–2754. [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel R. E., Lardy H. A. Influence of aurovertin on mitochondrial ATPase activity. J Biol Chem. 1975 Jul 10;250(13):4992–4995. [PubMed] [Google Scholar]
- Kagawa Y., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 8. Properties of a factor conferring oligomycin sensitivity on mitochondrial adenosine triphosphatase. J Biol Chem. 1966 May 25;241(10):2461–2466. [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- Lambeth D. O., Lardy H. A. Purification and properties of rat-liver-mitochondrial adenosine triphosphatase. Eur J Biochem. 1971 Oct 14;22(3):355–363. doi: 10.1111/j.1432-1033.1971.tb01552.x. [DOI] [PubMed] [Google Scholar]
- Layton D., Azzi A., Graziotti P. The use of the fluorescent probe aurovertin, to monitor energy linked conformational changes in mitochondrial ATPases. FEBS Lett. 1973 Oct 1;36(1):87–92. doi: 10.1016/0014-5793(73)80343-6. [DOI] [PubMed] [Google Scholar]
- Lee C., Ernster L. Studies of the energy-transfer system of submitochondrial particles. 2. Effects of oligomycin and aurovertin. Eur J Biochem. 1968 Feb;3(4):391–400. doi: 10.1111/j.1432-1033.1967.tb19542.x. [DOI] [PubMed] [Google Scholar]
- Lenaz G. Effect of aurovertin on energy-linked processes related to oxidative phosphorylation. Biochem Biophys Res Commun. 1965 Oct 26;21(2):170–175. doi: 10.1016/0006-291x(65)90104-x. [DOI] [PubMed] [Google Scholar]
- Linnett P. E., Mitchell A. D., Beechey R. B., Baum H. A reiteration of the equation derived by Easson & Stedman (1936) and its application to the inhibition of mitochondrial energy-linked functions by the aurovertins [proceedings]. Biochem Soc Trans. 1977;5(5):1510–1511. doi: 10.1042/bst0051510. [DOI] [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Roberton A. M., Beechey R. B., Holloway C. T., Knight I. G. The effect of aurovertin on a soluble mitochondrial adenosine triphosphatase. Biochem J. 1967 Sep;104(3):54C–55C. doi: 10.1042/bj1040054c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberton A. M., Holloway C. T., Knight I. G., Beechey R. B. A comparison of the effects of NN'-dicyclohexylcarbodi-imide, oligomycin A and aurovertin on enrgy-linked reactions in mitochondria and submitochondrial particles. Biochem J. 1968 Jul;108(3):445–456. doi: 10.1042/bj1080445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Stadt R. J., van Dam K. Binding of aurovertin to phosphorylating submitochondrial particles. Biochim Biophys Acta. 1974 May 22;347(2):253–263. doi: 10.1016/0005-2728(74)90049-8. [DOI] [PubMed] [Google Scholar]
- van de Stadt R. J., van Dam K., Slater E. C. Interaction of aurovertin with submitochondrial particles, deficient in ATPase inhibitor. Biochim Biophys Acta. 1974 May 22;347(2):224–239. doi: 10.1016/0005-2728(74)90047-4. [DOI] [PubMed] [Google Scholar]


