Abstract
The rate of DNA synthesis is exponentially growing cells was determined by isotopedilution analysis of the incorporation of [me-3H]thymidine. Thymidine concentrations greater than 7 micrometer were used so that the rate-limiting step governing incorporation would be at the level of DNA polymerase rather than at the level of thymidine kinase [Sjostrom & Forsdyke (1974) Biochem. J. 138, 253-262]. In early exponential phase the rate determined by isotope-dilution analysis closely correlated with the rates calculated either from growth curves or from known cell-cycle parameters. However, in late-exponential phase the rate calculated from the growth curve was less than that determined by isotope-dilution analysis. We conclude that, under certain conditions, the pool-corrected rate of incorporation of [me-3H]thymidine, as determined by isotope-dilution analysis, can accurately reflect the rate of DNA synthesis. Discrepancies between the observed rate of DNA synthesis and increase in cell number could reflect an exponential degeneration of post-S-phase cells.
Full text
PDF

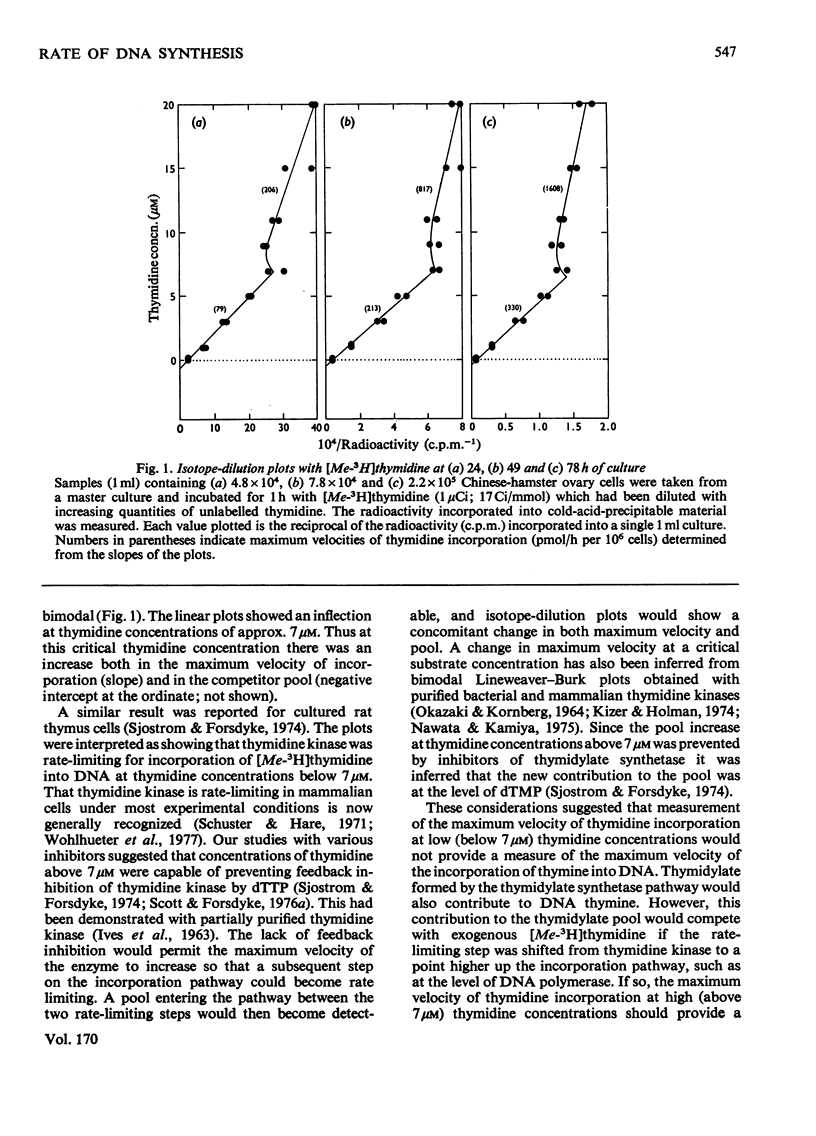
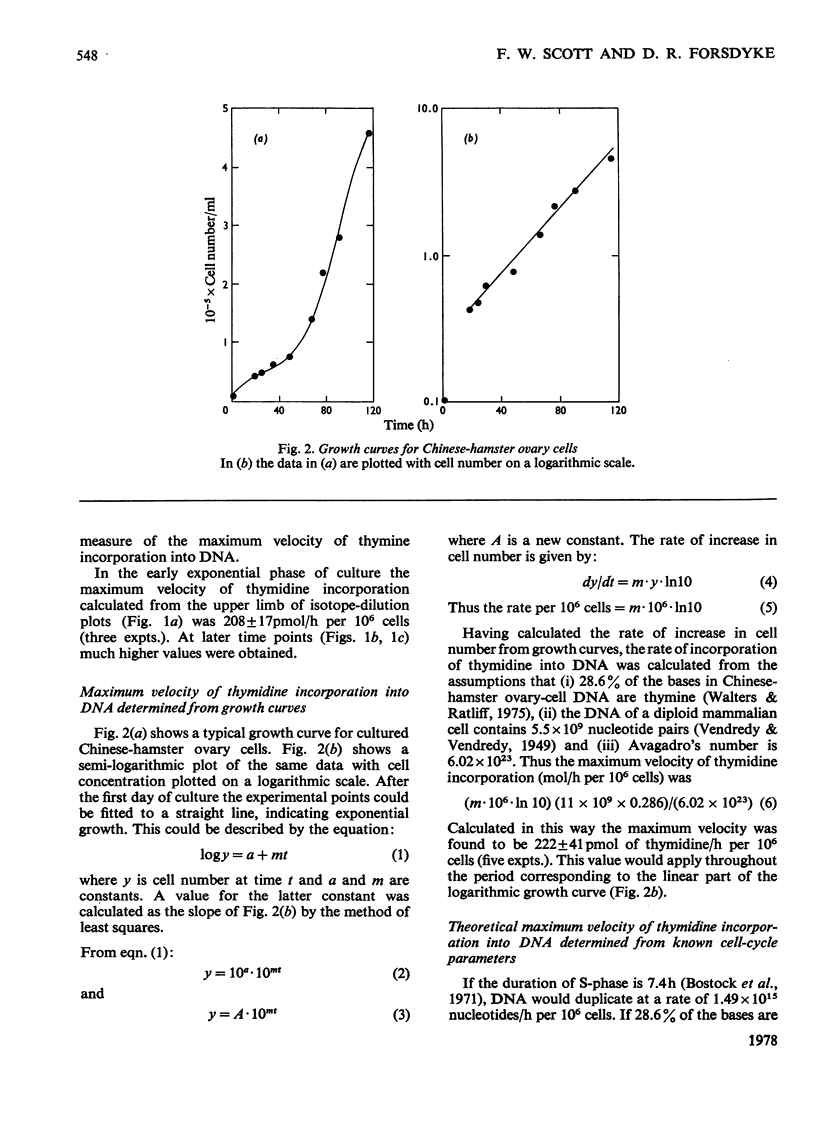

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostock C. J., Prescott D. M., Kirkpatrick J. B. An evaluation of the double thymidine block for synchronizing mammalian cells at the G1-S border. Exp Cell Res. 1971 Sep;68(1):163–168. doi: 10.1016/0014-4827(71)90599-4. [DOI] [PubMed] [Google Scholar]
- Chiu C. S., Tomich P. K., Greenberg G. R. Simultaneous initiation of synthesis of bacteriophage T4 DNA and of deoxyribonucleotides. Proc Natl Acad Sci U S A. 1976 Mar;73(3):757–761. doi: 10.1073/pnas.73.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsdyke D. R. Application of the isotope-dilution principle to the analysis of factors affecting the incorporation of (3H) uridine and (3H) cytidine into cultured lymphocytes. Evaluation of pools in serum and culture media. Biochem J. 1971 Dec;125(3):721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- Kizer D. E., Holman L. Purification and properties of thymidine kinase from regenerating rat liver. Biochim Biophys Acta. 1974 May 20;350(1):193–200. doi: 10.1016/0005-2744(74)90217-4. [DOI] [PubMed] [Google Scholar]
- Kuebbing D., Werner R. A model for compartmentation of de novo and salvage thymidine nucleotide pools in mammalian cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3333–3336. doi: 10.1073/pnas.72.9.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters C. J. Metabolic control and the microenvironment. Curr Top Cell Regul. 1977;12:75–105. doi: 10.1016/b978-0-12-152812-6.50009-3. [DOI] [PubMed] [Google Scholar]
- Meuth M., Aufreiter E., Reichard P. Deoxyribonucleotide pools in mouse-fibroblast cell lines with altered ribonucleotide reductase. Eur J Biochem. 1976 Dec;71(1):39–43. doi: 10.1111/j.1432-1033.1976.tb11087.x. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Moses V. The tentative identification in Escherichia coli of a multienzyme complex with glycolytic activity. Eur J Biochem. 1976 Jun 15;66(1):25–36. doi: 10.1111/j.1432-1033.1976.tb10421.x. [DOI] [PubMed] [Google Scholar]
- Nawata H., Kamiya T. Two molecular forms of thymidine kinase in the cytosol of regenerating rat liver. J Biochem. 1975 Dec;78(6):1215–1224. doi: 10.1093/oxfordjournals.jbchem.a131019. [DOI] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Reddy G. P., Singh A., Stafford M. E., Mathews C. K. Enzyme associations in T4 phage DNA precursor synthesis. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3152–3156. doi: 10.1073/pnas.74.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. Control of deoxyribonucleotide synthesis in vitro and in vivo. Adv Enzyme Regul. 1972;10:3–16. doi: 10.1016/0065-2571(72)90003-9. [DOI] [PubMed] [Google Scholar]
- Schuster G. S., Hare J. D. The role of phosphorylation in the uptake of thymidine in mammalian cells. In Vitro. 1971 May-Jun;6(6):427–436. doi: 10.1007/BF02616044. [DOI] [PubMed] [Google Scholar]
- Sjostrom D. A., Forsdyke D. R. Isotope-dilution analysis of rate-limiting steps and pools affecting the incorporation of thymidine and deoxycytidine into cultured thymus cells. Biochem J. 1974 Feb;138(2):253–262. doi: 10.1042/bj1380253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Baker R. M. Isolation of mutants of cultured mammalian cells. Methods Cell Biol. 1973;6:209–281. doi: 10.1016/s0091-679x(08)60052-7. [DOI] [PubMed] [Google Scholar]
- Walters R. A., Ratliff R. L. Lack of specific correlation of the deoxycytidine triphosphate pool level with rate of DNA synthesis. Biochim Biophys Acta. 1975 Dec 19;414(3):221–230. doi: 10.1016/0005-2787(75)90161-6. [DOI] [PubMed] [Google Scholar]
- Wittes R. E., Kidwell W. R. A kinetic approach to the determination of the S phase pool size of thymidine triphosphate in exponentially growing mouse L cells. J Mol Biol. 1973 Aug 15;78(3):473–486. doi: 10.1016/0022-2836(73)90469-5. [DOI] [PubMed] [Google Scholar]
- Wohlhueter R. M., Marz R., Graff J. C., Plagemann P. G. The application of rapid kinetic techniques to the transport of thymidine and 3-O-Methylglucose into Mammalian cells in suspension culture. J Cell Physiol. 1976 Dec;89(4):605–612. doi: 10.1002/jcp.1040890417. [DOI] [PubMed] [Google Scholar]


