Abstract
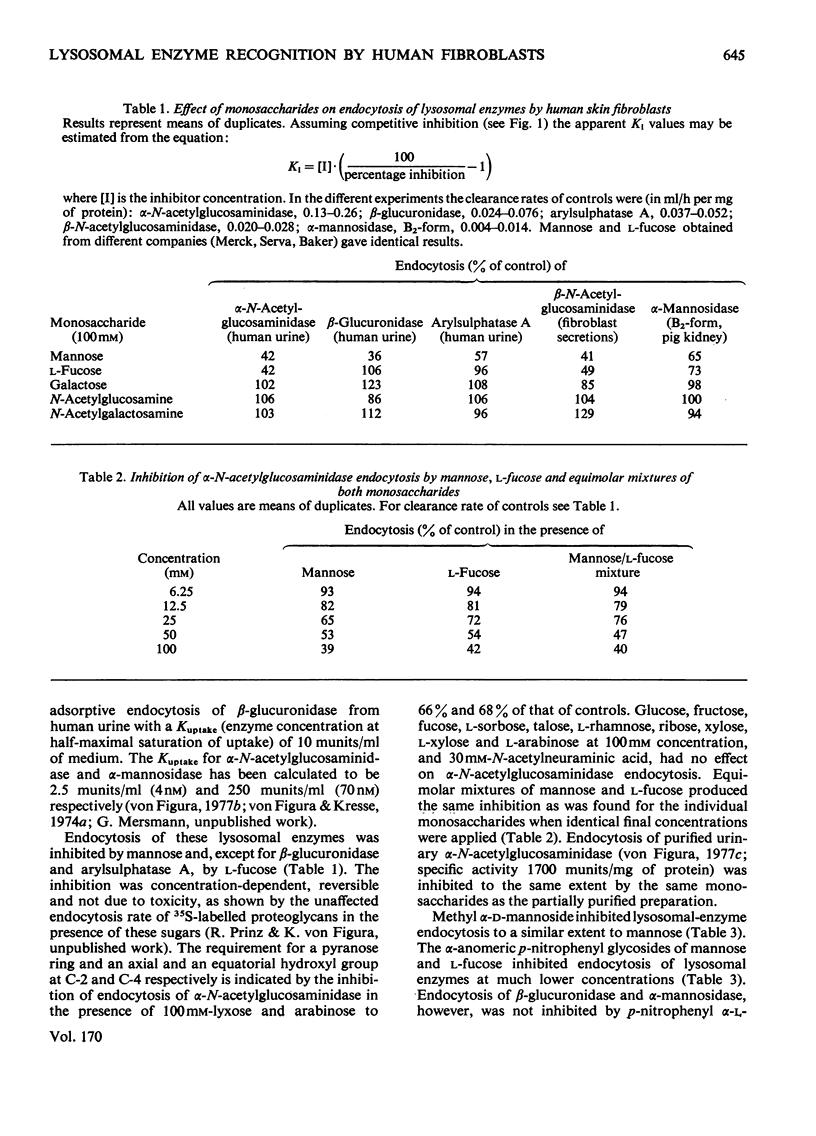
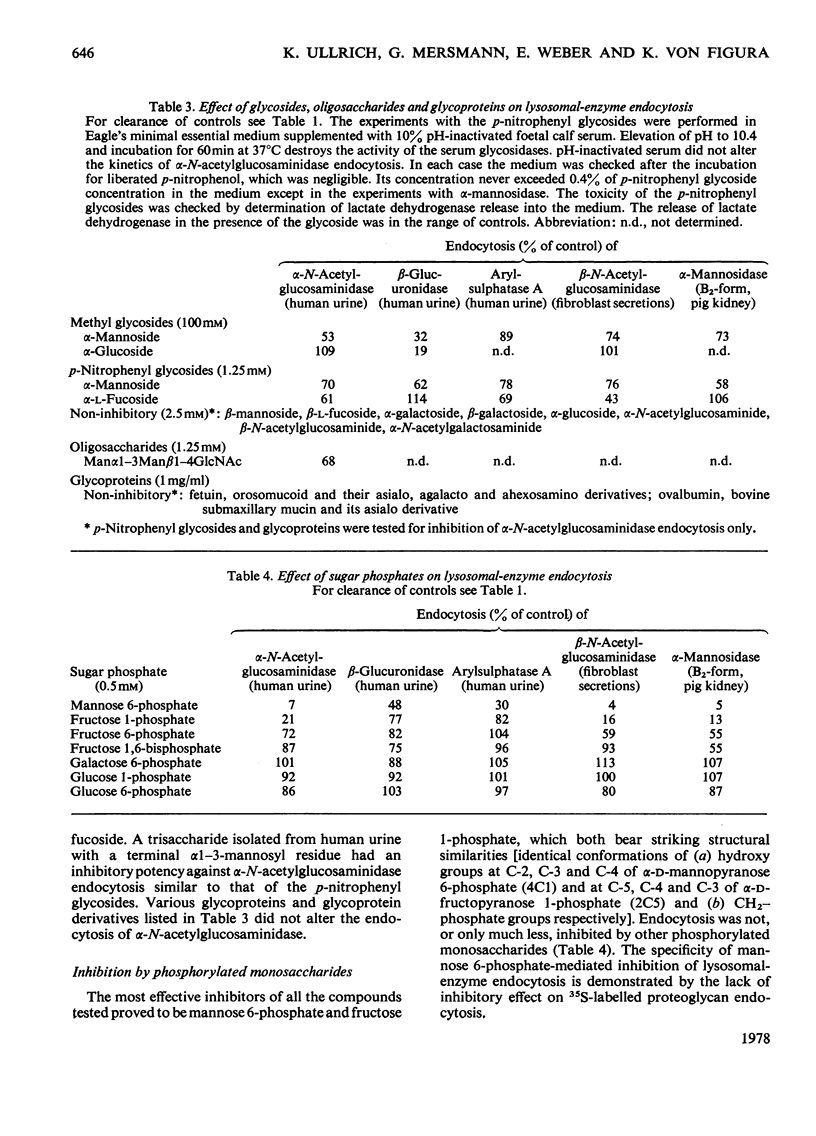
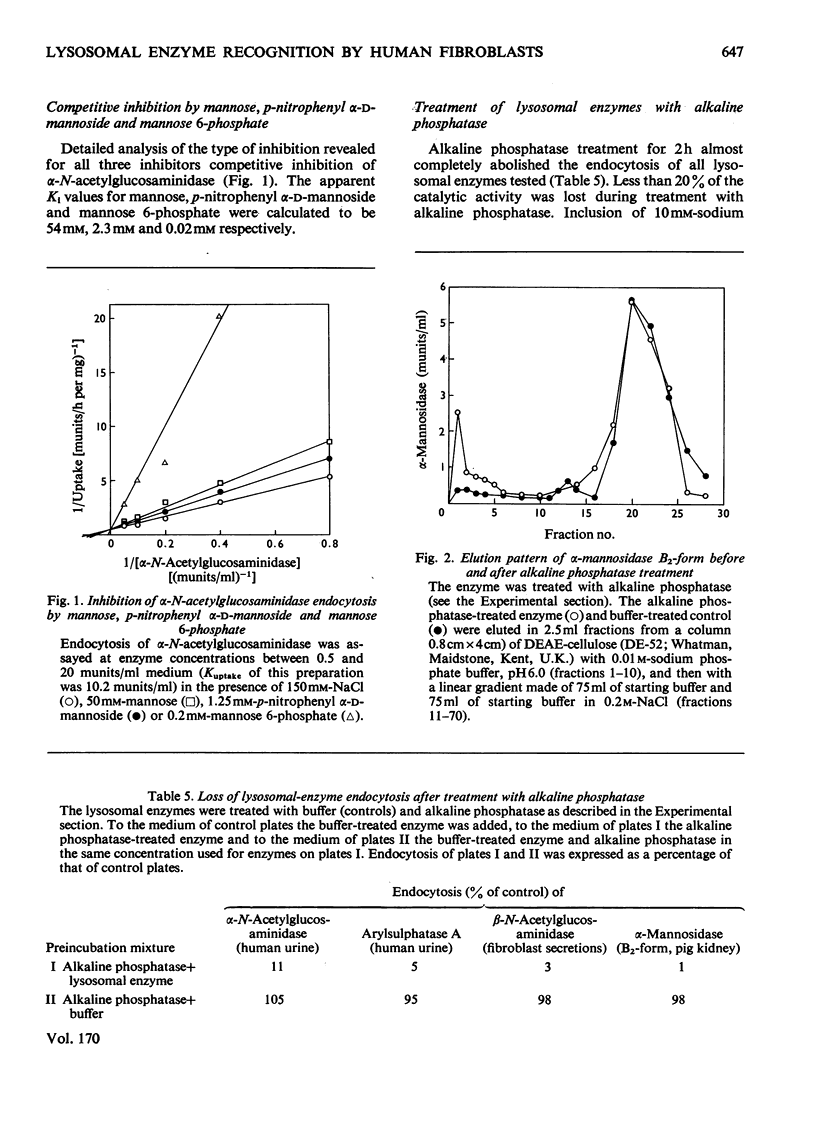
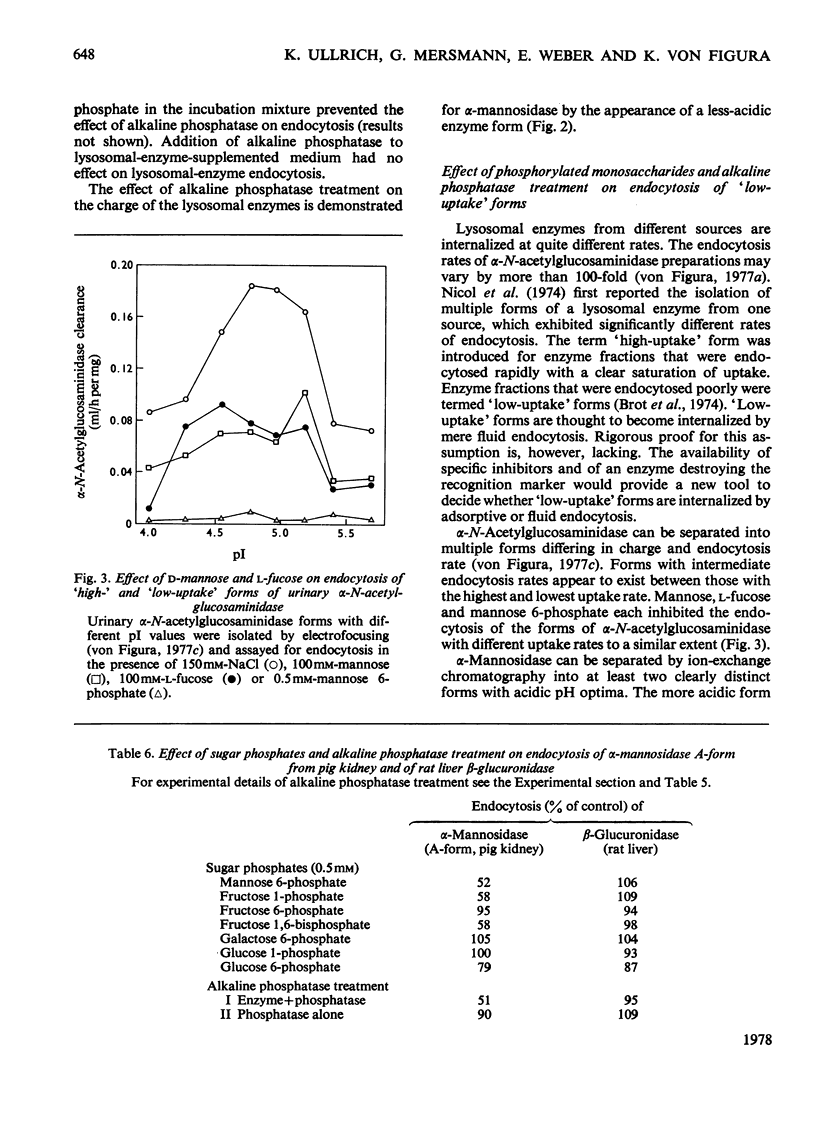
Adsorptive endocytosis of five different lysosomal enzymes from various human and non-human sources was susceptible to inhibition by mannose and l-fucose, methyl α-d-mannoside, α-anomeric p-nitrophenyl glycosides of mannose and l-fucose, mannose 6-phosphate and fructose 1-phosphate. A few exceptions from this general scheme were observed for particular enzymes, particularly for β-glucuronidase from human urine. The inhibition of α-N-acetylglucosaminidase endocytosis by mannose, p-nitrophenyl α-d-mannoside and mannose 6-phosphate was shown to be competitive. The loss of endocytosis after alkaline phosphatase treatment of lysosomal enzymes supports the hypothesis that the phosphorylated sugars compete with a phosphorylated carbohydrate on the enzymes for binding to the cell-surface receptors [Kaplan, Achord & Sly (1977) Proc. Natl. Acad. Sci. U.S.A. 74, 2026–2030]. Endocytosis of `low-uptake' forms of α-N-acetylglucosaminidase and α-mannosidase was likewise susceptible to inhibition by sugar phosphates and by alkaline phosphatase treatment, suggesting that `low-uptake' forms are either contaminated with `high-uptake' forms or are internalized via the same route as `high-uptake' forms. The existence of an alternative route for adsorptive endocytosis of lysosomal enzymes is indicated by the unaffected adsorptive endocytosis of rat liver β-glucuronidase in the presence of phosphorylated sugars and after treatment with alkaline phosphatase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Baynes J. W., Wold F. Effect of glycosylation on the in vivo circulating half-life of ribonuclease. J Biol Chem. 1976 Oct 10;251(19):6016–6024. [PubMed] [Google Scholar]
- Brot F. E., Glaser J. H., Roozen K. J., Sly W. S., Stahl P. D. In vitro correction of deficient human fibroblasts by beta-glucuronidase from different human sources. Biochem Biophys Res Commun. 1974 Mar 15;57(1):1–8. doi: 10.1016/s0006-291x(74)80349-9. [DOI] [PubMed] [Google Scholar]
- Chester M. A., Lundblad A., Masson P. K. The relationship between different forms of human alpha-mannosidase. Biochim Biophys Acta. 1975 Jun 24;391(2):341–348. doi: 10.1016/0005-2744(75)90258-2. [DOI] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. I. PURIFICATION AND PROPERTIES OF A NEURAMINIDASE AND A BETA-GALACTOSIDASE. ACTION ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1535–1543. doi: 10.1021/bi00898a025. [DOI] [PubMed] [Google Scholar]
- HUGHES R. C., JEANLOZ R. W. THE EXTRACELLULAR GLYCOSIDASES OF DIPLOCOCCUS PNEUMONIAE. II. PURIFICATION AND PROPERTIES OF A BETA-N-ACETYLGLUCOSAMINIDASE. ACTION ON A DERIVATIVE ON THE ALPHA-1-ACID GLYCOPROTEIN OF HUMAN PLASMA. Biochemistry. 1964 Oct;3:1543–1548. doi: 10.1021/bi00898a026. [DOI] [PubMed] [Google Scholar]
- Hickman S., Neufeld E. F. A hypothesis for I-cell disease: defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972 Nov 15;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- Hickman S., Shapiro L. J., Neufeld E. F. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974 Mar 15;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- Hieber V., Distler J., Myerowitz R., Schmickel R. D., Jourdian G. W. The role of glycosidically bound mannose in the assimilation of beta-galactosidase by generalized gangliosidosis fibroblasts. Biochem Biophys Res Commun. 1976 Dec 6;73(3):710–717. doi: 10.1016/0006-291x(76)90868-8. [DOI] [PubMed] [Google Scholar]
- Himeno Mnishimura Y., Tsuji H., Kato K. Purification and characterization of microsomal and lysosomal beta-glucuronidase from rat liver by use of immunoaffinity chromatography. Eur J Biochem. 1976 Nov 15;70(2):349–359. doi: 10.1111/j.1432-1033.1976.tb11024.x. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Achord D. T., Sly W. S. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci U S A. 1977 May;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff D., Nicol D. M., Pritzi P. Uptake of beta-glucuronidase by deficient human fibroblasts. Lab Invest. 1973 Oct;29(4):449–453. [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersmann G., Von Figura K., Buddecke E. Storage of mannose-containing material in cultured human mannosidosis cells and metabolic correction by pig kidney alpha-mannosidase. Hoppe Seylers Z Physiol Chem. 1976 May;357(5):641–648. doi: 10.1515/bchm2.1976.357.1.641. [DOI] [PubMed] [Google Scholar]
- Neufeld E. F., Lim T. W., Shapiro L. J. Inherited disorders of lysosomal metabolism. Annu Rev Biochem. 1975;44:357–376. doi: 10.1146/annurev.bi.44.070175.002041. [DOI] [PubMed] [Google Scholar]
- Nicol D. M., Lagunoff D., Pritzl P. Differential uptake of human beta-glucuronidase isoenzymes from spleen by deficient fibroblasts. Biochem Biophys Res Commun. 1974 Aug 5;59(3):941–946. doi: 10.1016/s0006-291x(74)80070-7. [DOI] [PubMed] [Google Scholar]
- Porter M. T., Fluharty A. L., Kihara H. Metachromatic leukodystrophy: arylsulfatase-A deficiency in skin fibroblast cultures. Proc Natl Acad Sci U S A. 1969 Mar;62(3):887–891. doi: 10.1073/pnas.62.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando G. N., Neufeld E. F. Recognition and receptor-mediated uptake of a lysosomal enzyme, alpha-l-iduronidase, by cultured human fibroblasts. Cell. 1977 Nov;12(3):619–627. doi: 10.1016/0092-8674(77)90262-8. [DOI] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Rodman J. S., Doebber T. Recognition of lysosomal glycosidases in vivo inhibited by modified glycoproteins. Nature. 1976 Nov 4;264(5581):86–88. doi: 10.1038/264086a0. [DOI] [PubMed] [Google Scholar]
- Stahl P., Six H., Rodman J. S., Schlesinger P., Tulsiani D. R., Touster O. Evidence for specific recognition sites mediating clearance of lysosomal enzymes in vivo. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4045–4049. doi: 10.1073/pnas.73.11.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Wynn C. H. The turnover of hamster fibroblast lysosomal beta-D-glucuronidase. Biochem J. 1977 Jan 15;162(1):201–203. doi: 10.1042/bj1620201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann U. N., Herschkowitz N. N. Studies on the pathogenetic mechanism of I-cell disease in cultured fibroblasts. Pediatr Res. 1974 Nov;8(11):865–869. doi: 10.1203/00006450-197411000-00002. [DOI] [PubMed] [Google Scholar]
- Winkelhake J. L., Nicolson G. L. Aglycosylantibody. Effects of exoglycosidase treatments on autochthonous antibody survival time in the circulation. J Biol Chem. 1976 Feb 25;251(4):1074–1080. [PubMed] [Google Scholar]
- von Figura K. Human alpha-N-acetylglucosaminidase. 1. Purification and properties. Eur J Biochem. 1977 Nov 1;80(2):523–533. [PubMed] [Google Scholar]
- von Figura K. Human alpha-n-acetylglucosaminidase. 2. Activity towards natural substrates and multiple recognition forms. Eur J Biochem. 1977 Nov 1;80(2):535–542. doi: 10.1111/j.1432-1033.1977.tb11909.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Kresse H. Inhibition of pinocytosis by cytochalasin B. Decrease in intracellular lysosomal-enzyme activities and increased storage of glycosaminoglycans. Eur J Biochem. 1974 Oct 2;48(2):357–363. doi: 10.1111/j.1432-1033.1974.tb03777.x. [DOI] [PubMed] [Google Scholar]
- von Figura K., Kresse H. Quantitative aspects of pinocytosis and the intracellular fate of N-acetyl-alpha-D-glucosaminidase in Sanfilippo B fibroblasts. J Clin Invest. 1974 Jan;53(1):85–90. doi: 10.1172/JCI107563. [DOI] [PMC free article] [PubMed] [Google Scholar]


