Abstract
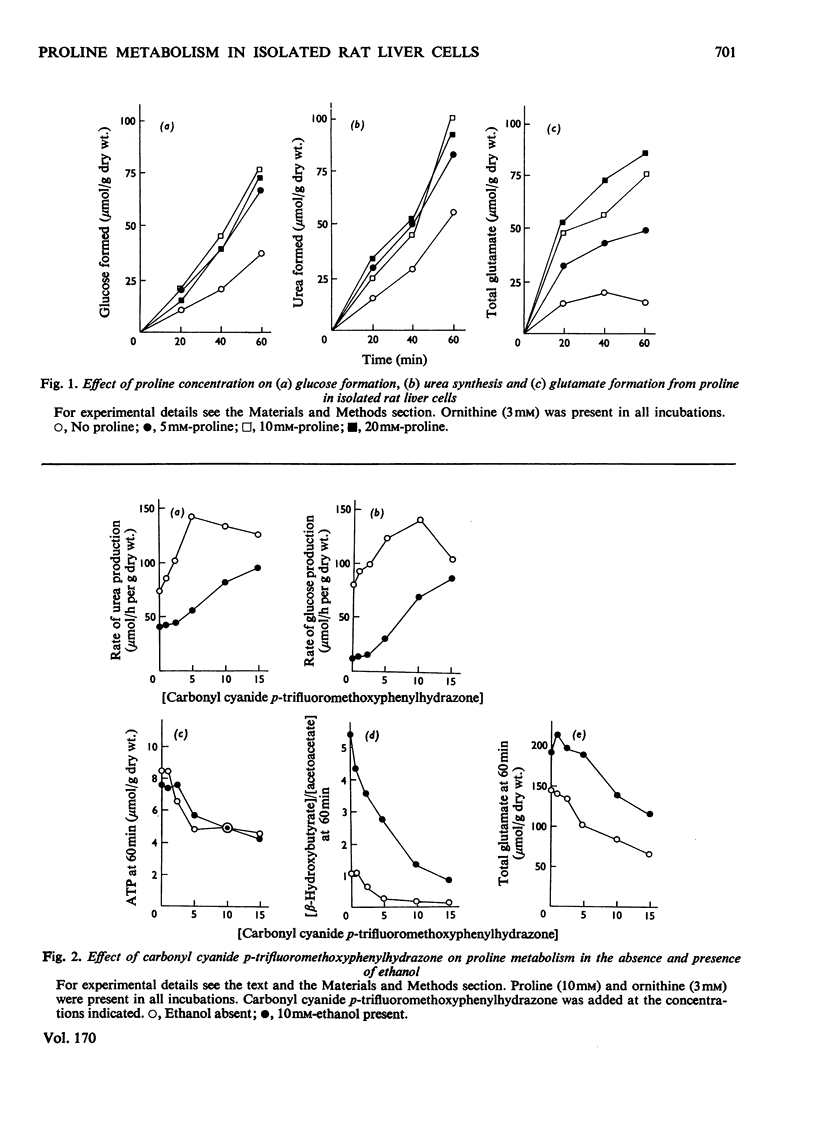
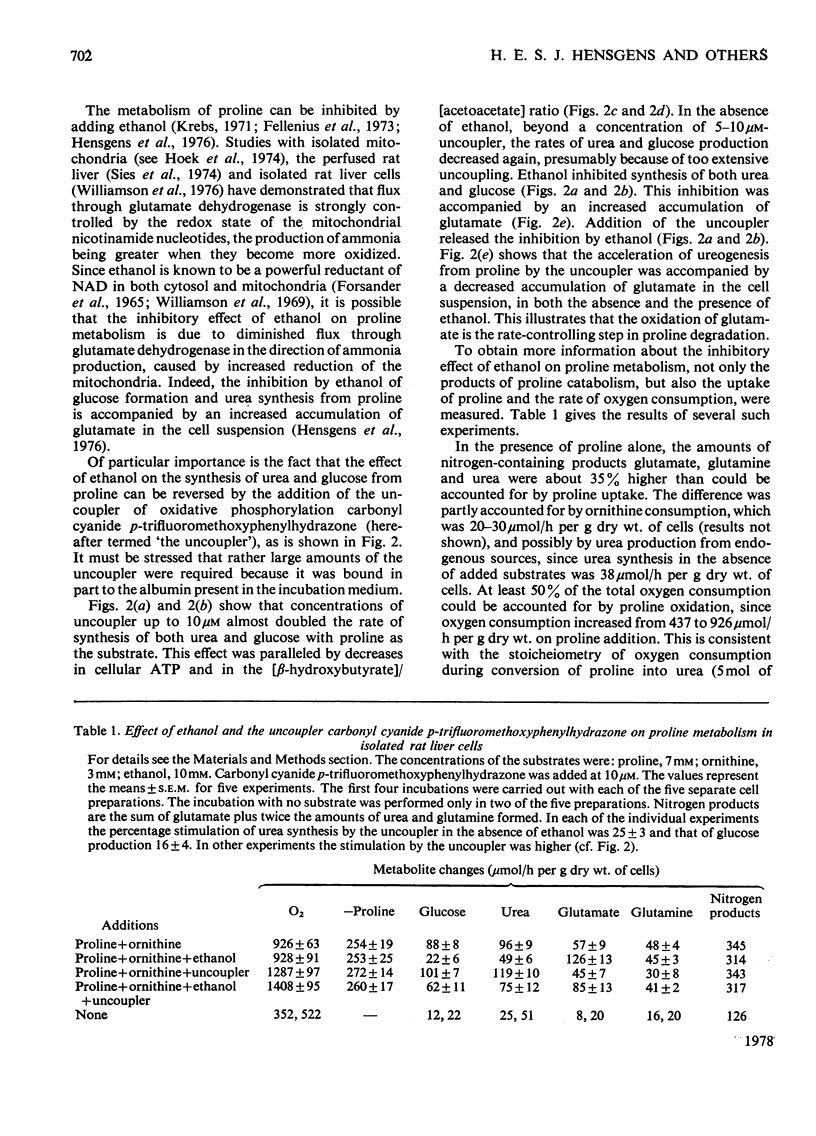
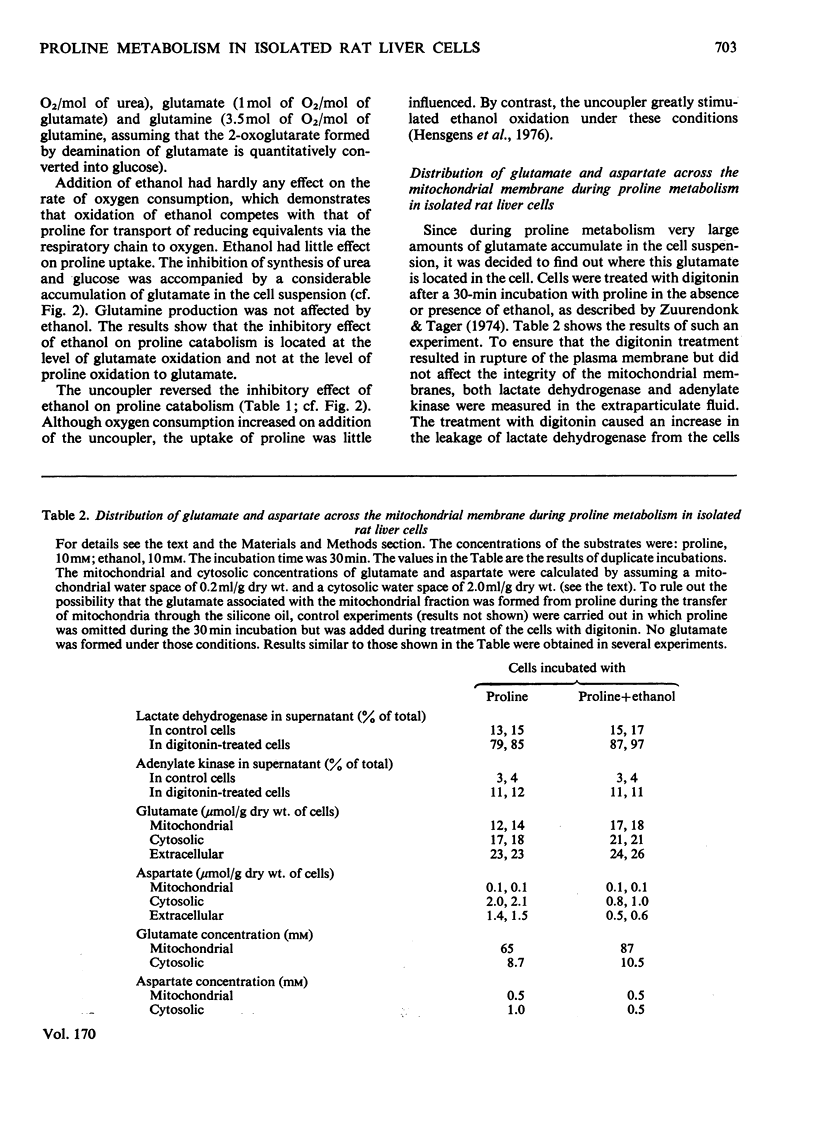
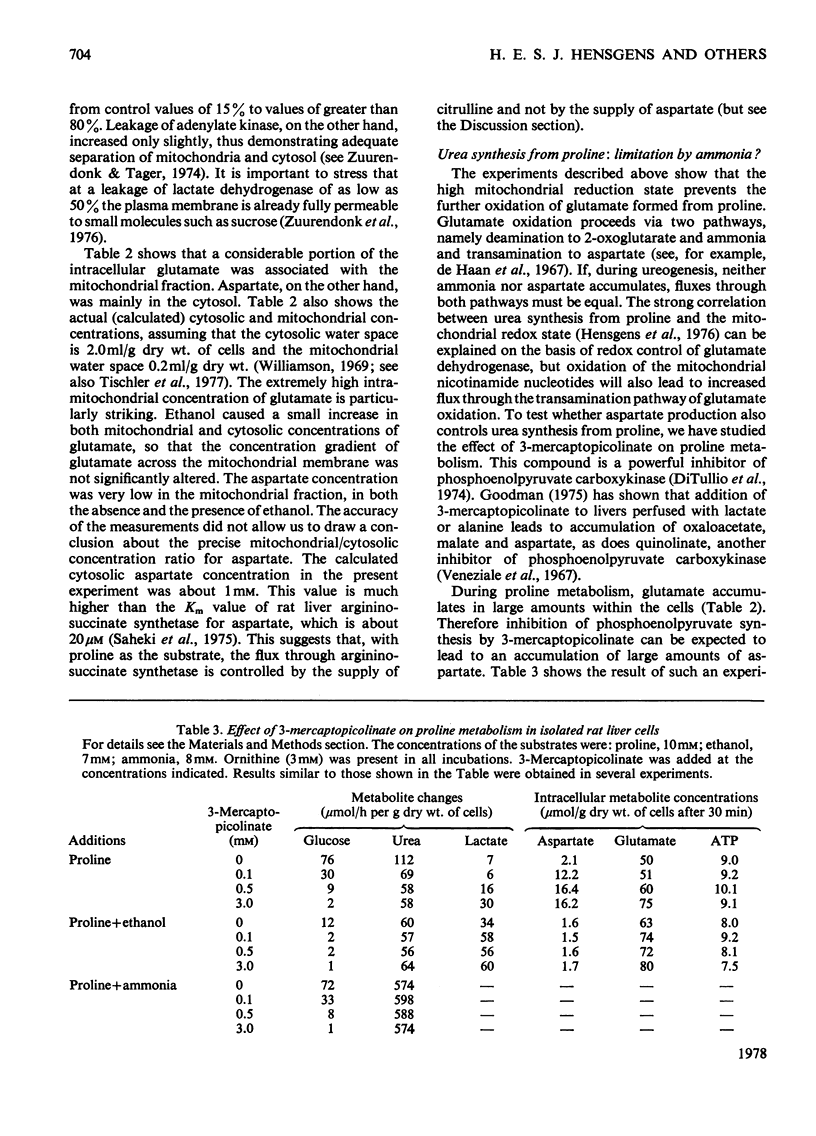
The metabolism of proline was studied in liver cells isolated from starved rats. The following observations were made. 1. Consumption of proline could be largely accounted for by production of glucose, urea, glutamate and glutamine. 2. At least 50% of the total consumption of oxygen was used for proline catabolism. 3. Ureogenesis and gluconeogenesis from proline could be stimulated by partial uncoupling of oxidative phosphorylation. 4. Addition of ethanol had little effect on either proline uptake or oxygen consumption, but strongly inhibited the production of both urea and glucose and caused further accumulation of glutamate and lactate. Accumulation of glutamine was not affected by ethanol. 5. The effects of ethanol could be overcome by partial uncoupling of oxidative phosphorylation. 6. The apparent Km values of argininosuccinate synthetase (EC 6.3.4.5) for aspartate and citrulline in the intact hepatocyte are higher than those reported for the isolated enzyme. 7. 3-Mercaptopicolinate, an inhibitor of phosphoenolpyruvate carboxykinase (EC 4.1.1.32), greatly enhanced cytosolic aspartate accumulation during proline metabolism, but inhibited urea synthesis. 8. It is concluded that when proline is provided as a source of nitrogen to liver cells, production of ammonia by oxidative deamination of glutamate is inhibited by the highly reduced state of the nicotinamide nucleotides within the mitochondria. 9. Conversion of proline into glucose and urea is a net-energy-yielding process, and the high state of reduction of the nicotinamide nucleotides is presumably maintained by a high phosphorylation potential. Thus when proline is present as sole substrate, the further oxidation of glutamate by glutamate dehydrogenase (EC 1.4.1.3) is limited by the rate of energy expenditure of the cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Holloway P. A., Aberti K. G. The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem J. 1975 Jun;148(3):353–362. doi: 10.1042/bj1480353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner G., Neupert W. Localisation of proline oxidase and Delta-pyrroline-5-carboxylic acid dehydrogenase in rat liver. FEBS Lett. 1969 Jun;3(4):283–286. doi: 10.1016/0014-5793(69)80159-6. [DOI] [PubMed] [Google Scholar]
- DiTullio N. W., Berkoff C. E., Blank B., Kostos V., Stack E. J., Saunders H. L. 3-mercaptopicolinic acid, an inhibitor of gluconeogenesis. Biochem J. 1974 Mar;138(3):387–394. doi: 10.1042/bj1380387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott K. R., Tipton K. F. Kinetic studies of bovine liver carbamoyl phosphate synthetase. Biochem J. 1974 Sep;141(3):807–816. doi: 10.1042/bj1410807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROSANDER O. A., RAEIHAE N., SALASPURO M., MAEENPAEAE P. INFLUENCE OF ETHANOL ON THE LIVER METABOLISM OF FED AND STARVED RATS. Biochem J. 1965 Jan;94:259–265. doi: 10.1042/bj0940259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidmann B., Goodman E. H., Jr, Saunders H. L., Kostos V., Weinhouse S. An estimation of pyruvate recycling during gluconeogenesis in the perfused rat liver. Arch Biochem Biophys. 1971 Apr;143(2):566–578. doi: 10.1016/0003-9861(71)90241-4. [DOI] [PubMed] [Google Scholar]
- Goodman M. N. Effect of 3-mercaptopicolinic acid on gluconeogenesis and gluconeogenic metabolite concentrations in the isolated perfused rat liver. Biochem J. 1975 Jul;150(1):137–139. doi: 10.1042/bj1500137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Stubbs M., Krebs H. A. Restricted permeability of rat liver for glutamate and succinate. Biochem J. 1968 May;107(6):807–815. doi: 10.1042/bj1070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J. B., Ernster L., de Haan E. J., Tager J. M. The nicotinamide nucleotide specificity of glutamate dehydrogenase in intact rat-liver mitochondria. Biochim Biophys Acta. 1974 Mar 26;333(3):546–559. doi: 10.1016/0005-2728(74)90138-8. [DOI] [PubMed] [Google Scholar]
- Hunninghake D., Grisolia S. A sensitive and convenient micromethod for estimation of urea, citrulline, and carbamyl derivatives. Anal Biochem. 1966 Aug;16(2):200–205. doi: 10.1016/0003-2697(66)90147-3. [DOI] [PubMed] [Google Scholar]
- JOHNSON A. B., STRECKER H. J. The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J Biol Chem. 1962 Jun;237:1876–1882. [PubMed] [Google Scholar]
- Katz N., Jungermann K. Autoregulatory shift from fructolysis to lactate gluconeogenisis in rat hepatocyte suspensions. The problem of metabolic zonation of liver parenchyma. Hoppe Seylers Z Physiol Chem. 1976 Mar;357(3):359–375. doi: 10.1515/bchm2.1976.357.1.359. [DOI] [PubMed] [Google Scholar]
- McGivan J. D., Chappell J. B. On the metabolic function of glutamate dehydrogenase in rat liver. FEBS Lett. 1975 Mar 15;52(1):1–7. doi: 10.1016/0014-5793(75)80624-7. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G. A., Tager J. M., Williamson J. R. Role of anion translocation across the mitochondrial membrane in the regulation of urea synthesis from ammonia by isolated rat hepatocytes. J Biol Chem. 1975 Oct 10;250(19):7728–7738. [PubMed] [Google Scholar]
- PAMILJANS V., KRISHNASWAMY P. R., DUMVILLE G., MEISTER A. Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry. 1962 Jan;1:153–158. doi: 10.1021/bi00907a023. [DOI] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Gluconeogenesis in the kidney cortex. Quantitative estimation of carbon flow. J Biol Chem. 1972 Oct 10;247(19):6047–6054. [PubMed] [Google Scholar]
- Saheki T., Kusumi T., Takada S., Katsunuma T., Katunuma N. Crystallization and some properties of argininosuccinate synthase from rat liver. FEBS Lett. 1975 Oct 15;58(1):314–317. doi: 10.1016/0014-5793(75)80287-0. [DOI] [PubMed] [Google Scholar]
- Sies H., Häussinger D., Grosskopf M. Mitochondrial nicotinamide nucleotide systems: ammonium chloride responses and associated metabolic transitions in hemoglobin-free perfused rat liver. Hoppe Seylers Z Physiol Chem. 1974 Mar;355(3):305–320. doi: 10.1515/bchm2.1974.355.1.305. [DOI] [PubMed] [Google Scholar]
- Siess E. A., Wieland O. H. Phosphorylation state of cytosolic and mitochondrial adenine nucleotides and of pyruvate dehydrogenase in isolated rat liver cells. Biochem J. 1976 Apr 15;156(1):91–102. doi: 10.1042/bj1560091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Regulation of rat liver glutamine synthetase: activation by alpha-ketoglutarate and inhibition by glycine, alanine, and carbamyl phosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):781–785. doi: 10.1073/pnas.68.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- Veneziale C. M., Walter P., Kneer N., Lardy H. A. Influence of L-tryptophan and its metabolites on gluconeogenesis in the isolated, perfused liver. Biochemistry. 1967 Jul;6(7):2129–2138. doi: 10.1021/bi00859a034. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]


