Abstract
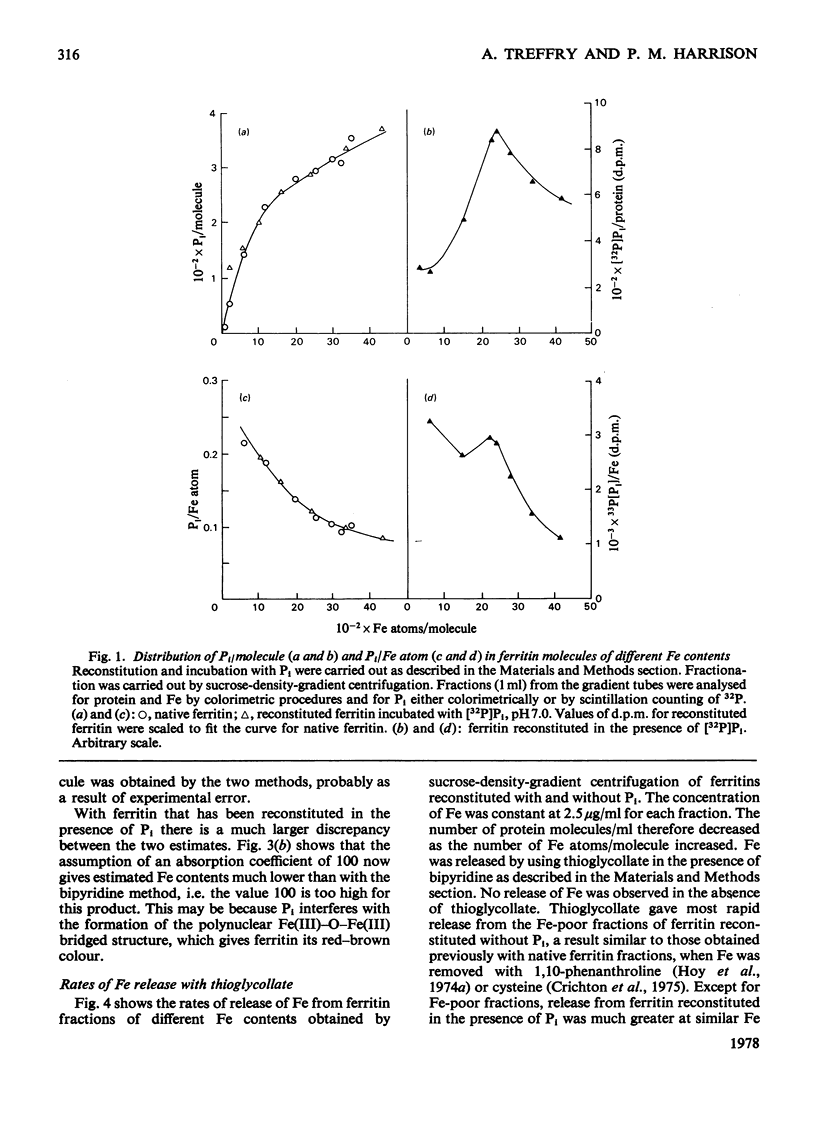
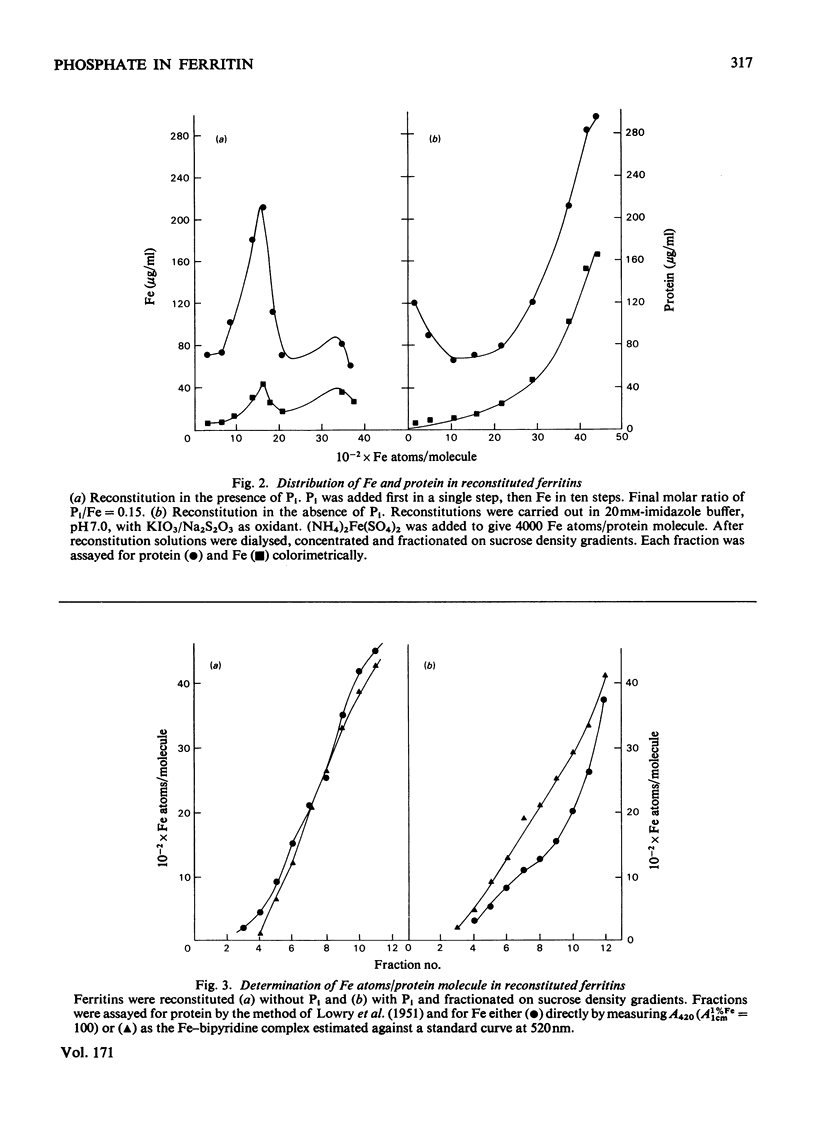
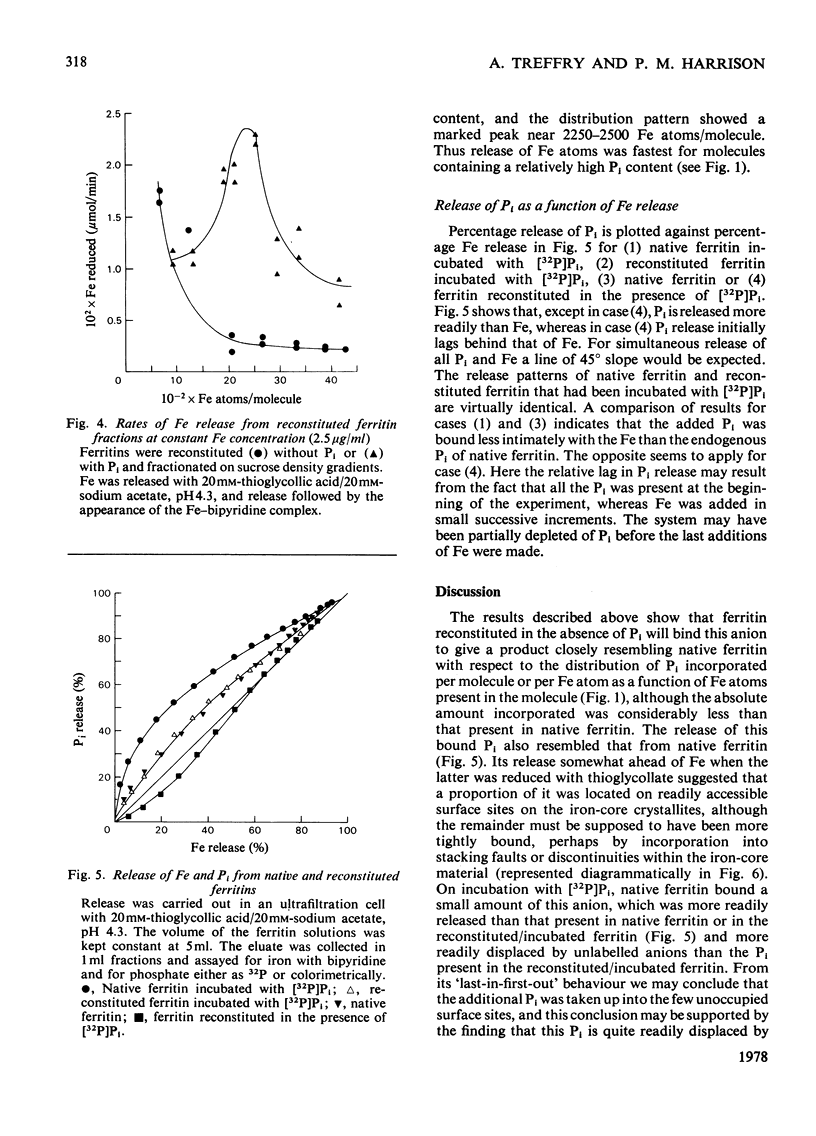
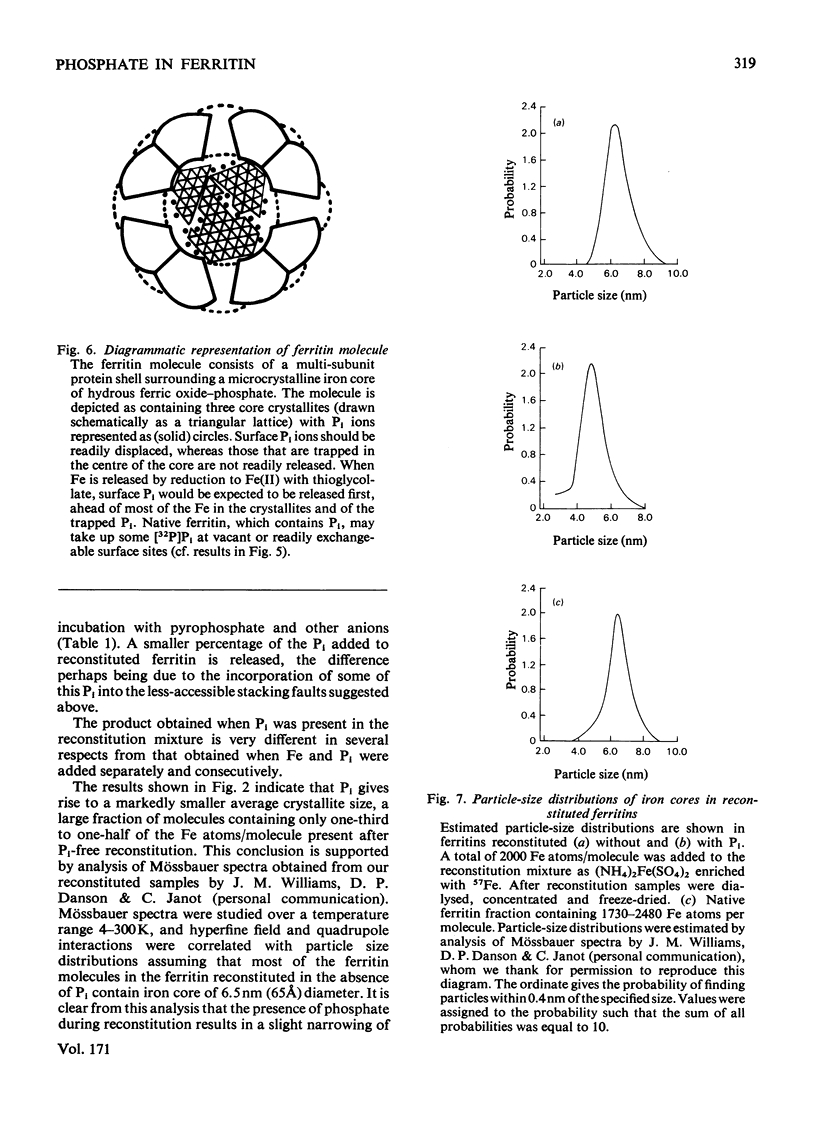
When ferritin is reconstituted from Fe and apoferritin in vitro in the presence of Pi, the product obtained differs both from native ferritin and from ferritin reconstituted in the absence of Pi. When the latter is incubated with Pi the product resembles native ferritin with respect both to the pattern of Pi incorporated per molecule or per Fe atom and to the ease of release of this Pi relative to Fe release. It is concluded that much of the Pi of native ferritin is adsorbed on surfaces of ferritin iron-core crystallites. The results also suggest that Pi is not present at the intracellular site of Fe incorporation into ferritin, but is added after Fe.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anner B., Mossmayer M. Rapid determination of inorganic phosphate in biological systems by a highly sensitive photometric method. Anal Biochem. 1975 May 12;65(1-2):305–309. doi: 10.1016/0003-2697(75)90514-x. [DOI] [PubMed] [Google Scholar]
- DRYSDALE J. W., MUNRO H. N. SMALL-SCALE ISOLATION OF FERRITIN FOR THE ASSAY OF THE INCORPORATION OF 14C-LABELLED AMINO ACIDS. Biochem J. 1965 Jun;95:851–858. doi: 10.1042/bj0950851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARRANT J. L. An electron microscopic study of ferritin. Biochim Biophys Acta. 1954 Apr;13(4):569–576. doi: 10.1016/0006-3002(54)90376-5. [DOI] [PubMed] [Google Scholar]
- Fischbach F. A., Harrison P. M., Hoy T. G. The structural relationship between ferritin protein and its mineral core. J Mol Biol. 1969 Jan 14;39(1):235–238. doi: 10.1016/0022-2836(69)90344-1. [DOI] [PubMed] [Google Scholar]
- Haggis G. H. The iron oxide core of the ferritin molecule. J Mol Biol. 1965 Dec;14(2):598–602. doi: 10.1016/s0022-2836(65)80210-8. [DOI] [PubMed] [Google Scholar]
- Harrison P. M. Ferritin: an iron-storage molecule. Semin Hematol. 1977 Jan;14(1):55–70. [PubMed] [Google Scholar]
- Harrison P. M., Fischbach F. A., Hoy T. G., Haggis G. H. Ferric oxyhydroxide core of ferritin. Nature. 1967 Dec 23;216(5121):1188–1190. doi: 10.1038/2161188a0. [DOI] [PubMed] [Google Scholar]
- Hoare R. J., Harrison P. M., Hoy T. G. Structure of horse-spleen apoferritin at 6 angstom resolution. Nature. 1975 Jun 19;255(5510):653–654. doi: 10.1038/255653a0. [DOI] [PubMed] [Google Scholar]
- Hoy T. G., Harrison P. M., Shabbir M., Macara I. G. The release of iron from horse spleen ferritin to 1,10-phenanthroline. Biochem J. 1974 Jan;137(1):67–70. doi: 10.1042/bj1370067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy T. G., Harrison P. M., Shabbir M. Uptake and release of ferritin iron. Surface effects and exchange within the crystalline core. Biochem J. 1974 Jun;139(3):603–607. doi: 10.1042/bj1390603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallner A. Determination of phosphate in serum and urine by a single step malachite-green method. Clin Chim Acta. 1975 Feb 22;59(1):35–39. doi: 10.1016/0009-8981(75)90215-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macara I. G., Hoy T. G., Harrison P. M. The formation of ferritin from apoferritin. Kinetics and mechanism of iron uptake. Biochem J. 1972 Jan;126(1):151–162. doi: 10.1042/bj1260151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massover W. H., Cowley J. M. The ultrastructure of ferritin macromolecules. The lattice structure of the core crystallites. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3847–3851. doi: 10.1073/pnas.70.12.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. C. Triton X-100 scintillant for carbon-14 labelled materials. Int J Appl Radiat Isot. 1968 Jul;19(7):557–563. doi: 10.1016/0020-708x(68)90065-3. [DOI] [PubMed] [Google Scholar]
- Van Kreel B. K., Pijnenburg A. M., Van Eijk H. G., Leijnse B. The incorporation of 59Fe,[3H]leucine and [32P]inorganic phosphate in ferritin during the perfusion of isolated rat livers. Biochim Biophys Acta. 1972 Jun 26;273(1):243–247. doi: 10.1016/0304-4165(72)90213-9. [DOI] [PubMed] [Google Scholar]


