Abstract
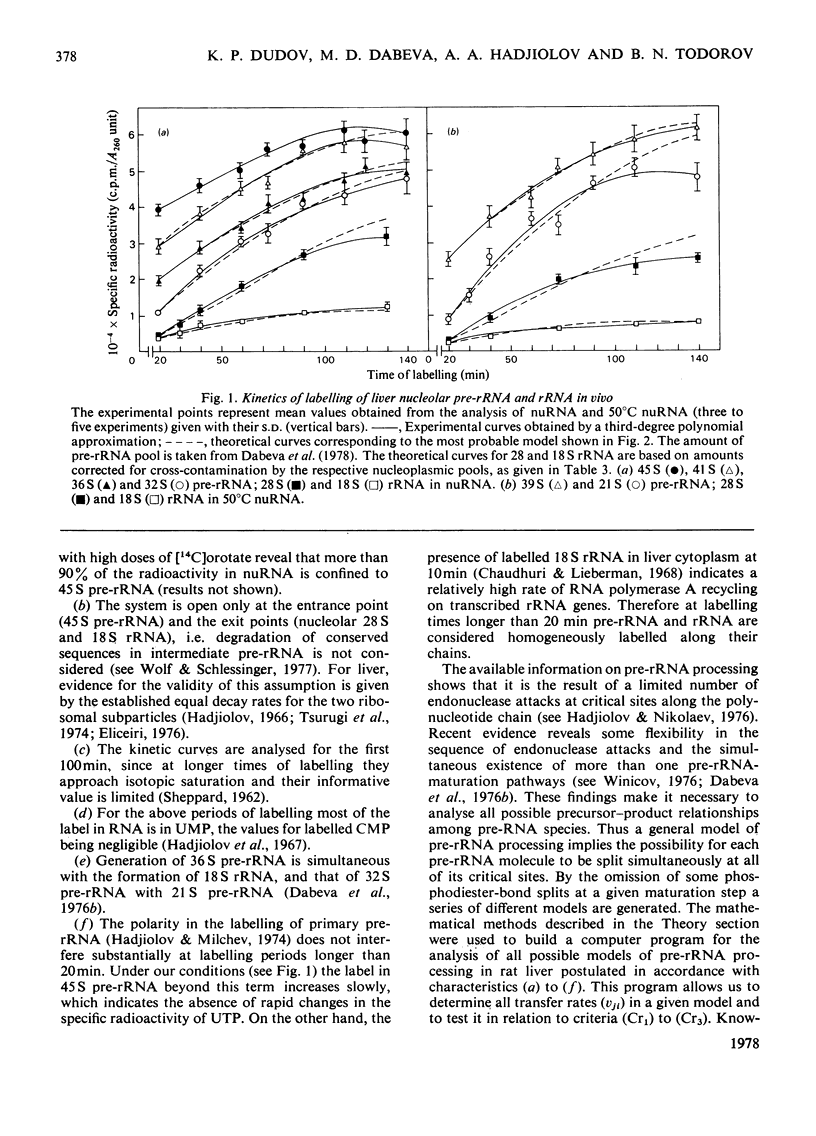
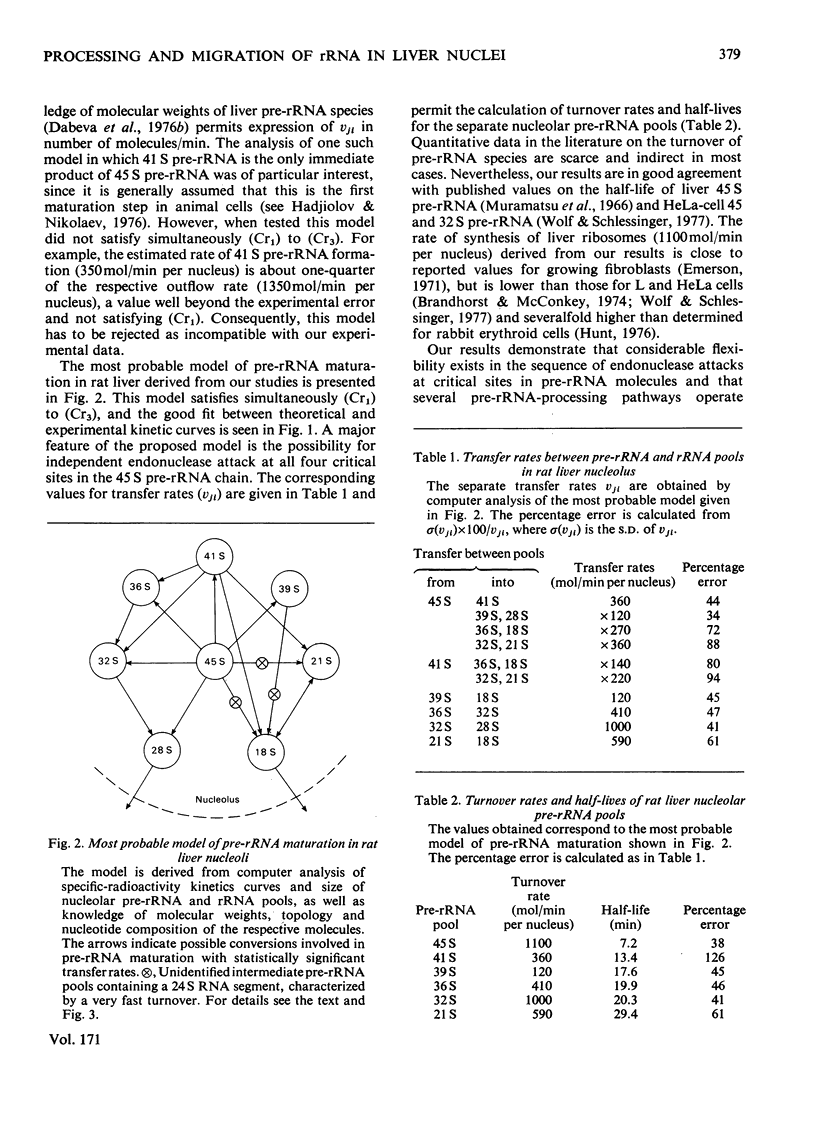
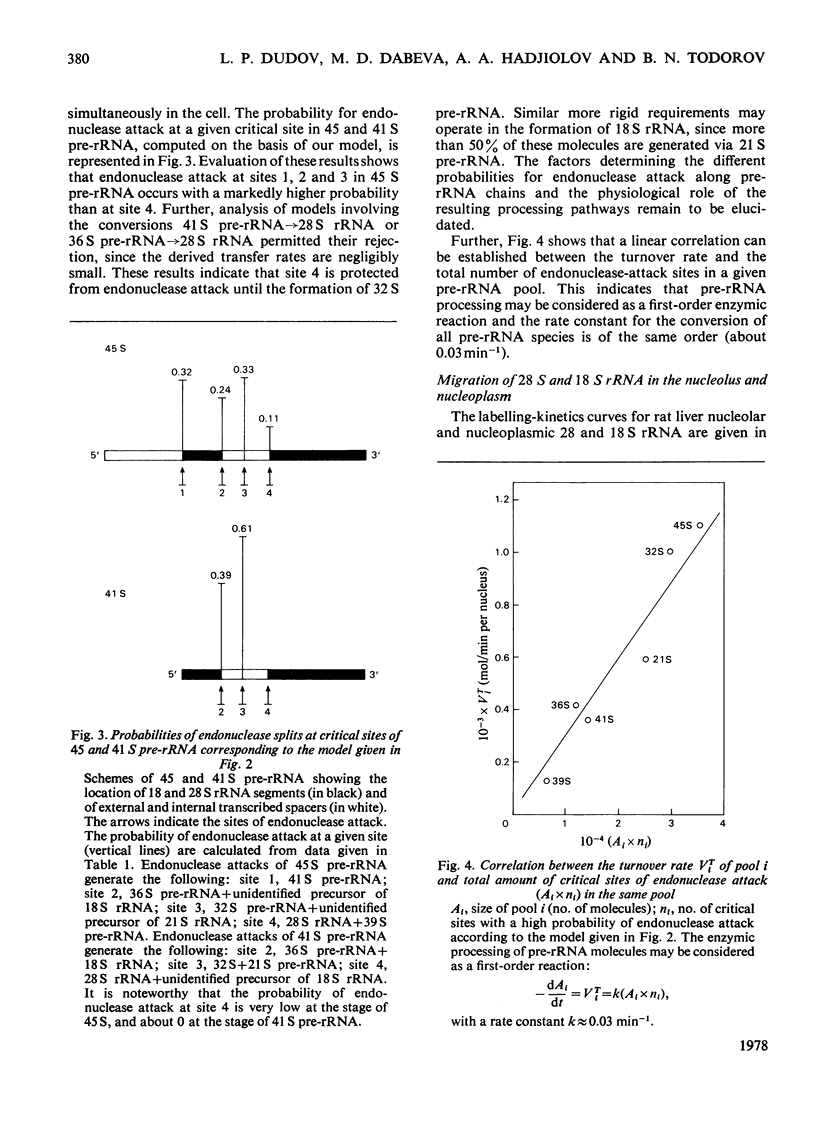
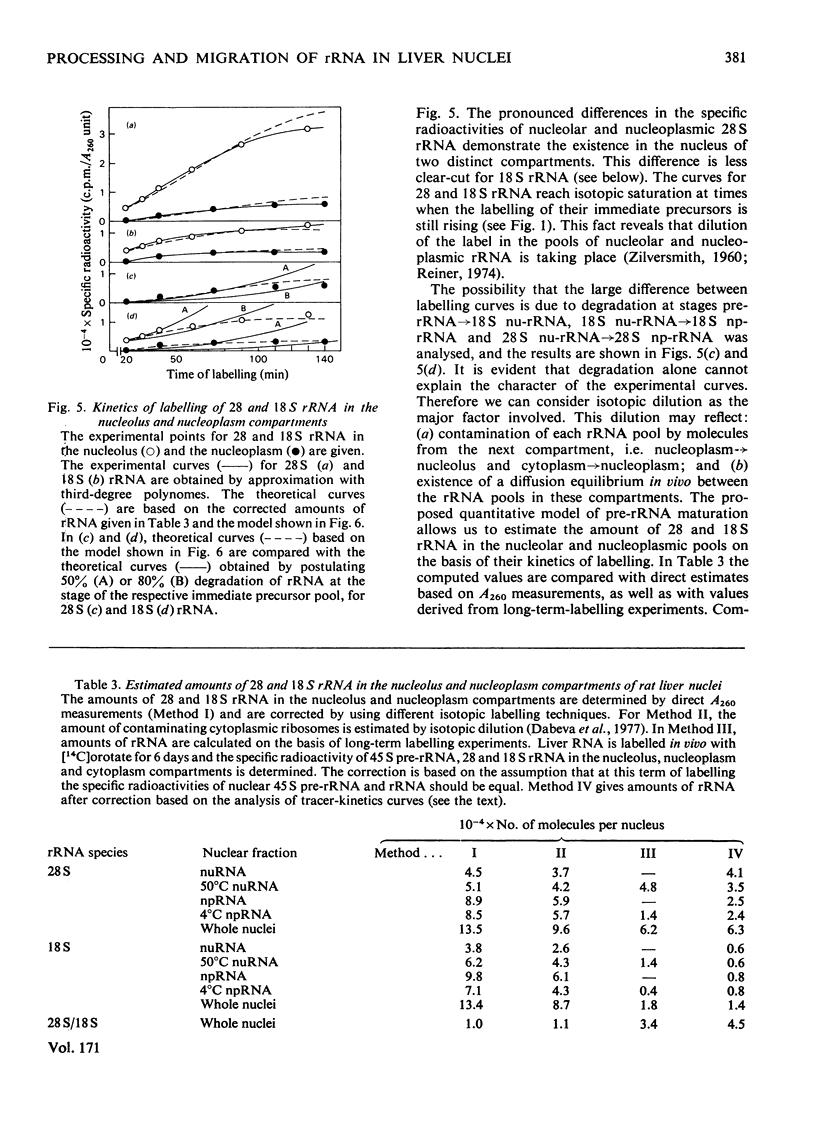
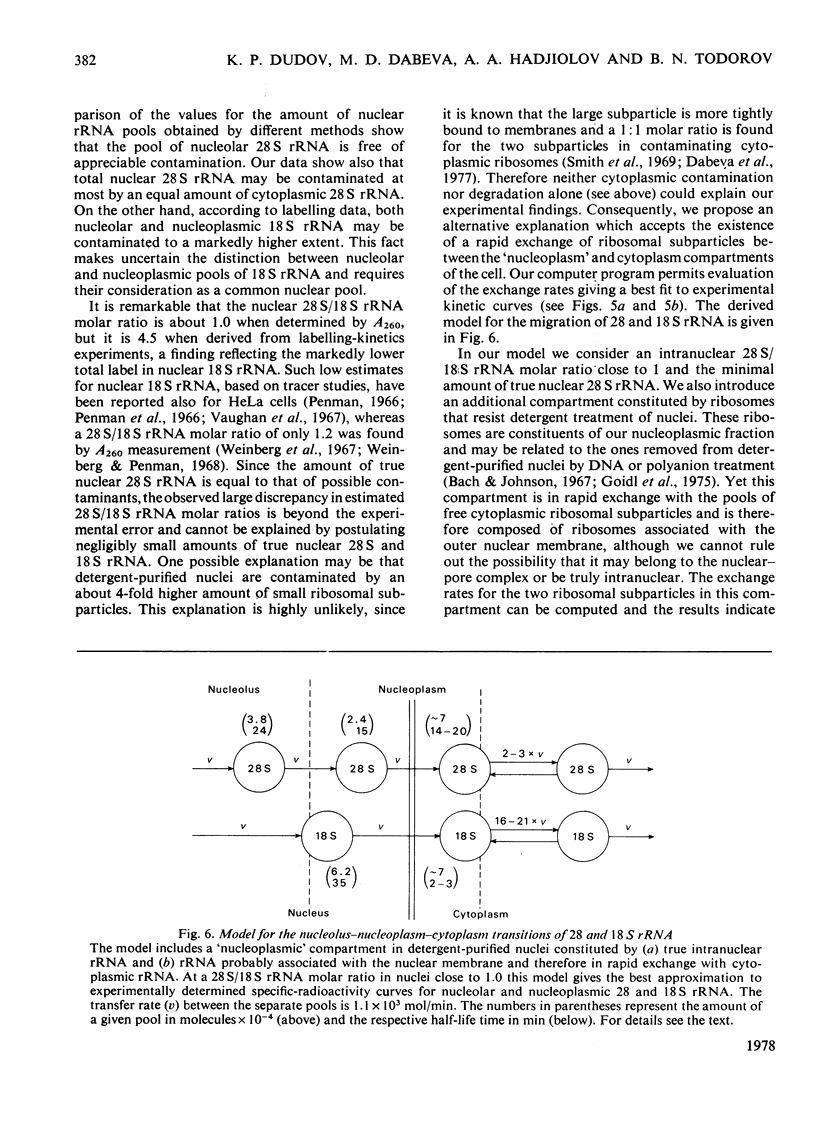
Kinetic studies on the labelling in vivo with [14C]orotate of rat liver nucleolar and nucleoplasmic pre-rRNA (precursor of rRNA) and rRNA, isolated from detergent-purified nuclei, were carried out. The mathematical methods used for the computer analysis of specific-radioactivity curves are described. Evaluation of the experimental data permitted the selection of the most probable models for the processing of pre-rRNA and the nucleo-cytoplasmic transfer of rRNA. It was shown that considerable flexibility exists in the sequence of endonuclease attacks at critical sites of 45 and 41 S pre-rRNA chains, resulting in the simultaneous occurrence of several processing pathways. However, the phosphodiester bonds involved in the formation of mature 28 and 18 S rRNA appear to be protected until the generation of their immediate pre-rRNA. The turnover rates and half-lives of all pre-rRNA and rRNA pools were determined. The turnover rate of 45 S pre-rRNA corresponds to the formation of 1100 ribosomes/min per nucleus. The model for the nucleolus-nucleoplasm-cytoplasm migration of rRNA includes a 'nucleoplasm' compartment in which the small ribosomal subparticle is in rapid equilibrium with the respective cytoplasmic pool. At equimolar amounts of nuclear 28 and 18 S rRNA this model explains the faster appearance of labelled small ribosomal subparticles in the cytoplasm simultaneous with a lower labelling of nuclear 18 S rRNA as compared with 28 S rRNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. L. Investigation of the effect of data error on the determination of physiological parameters by means of compartmental analysis. Biochem J. 1972 Apr;127(2):437–438. doi: 10.1042/bj1270437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M. K., Johnson H. G. The isolation and characterization of a novel microsome fraction from washed and detergent-treated nuclei of HeLa cells by the use of solutions containing deoxyribonucleic acid. Biochemistry. 1967 Jul;6(7):1916–1933. doi: 10.1021/bi00859a007. [DOI] [PubMed] [Google Scholar]
- Chaudhuri S., Lieberman I. Time required by the normal and regenerating rat liver cell to make a ribosome. J Mol Biol. 1968 Apr 14;33(1):323–326. doi: 10.1016/0022-2836(68)90299-4. [DOI] [PubMed] [Google Scholar]
- Dabeva M. D., Dudov K. P., Hadjiolov A. A., Emanuilov I., Todorov B. N. Intranuclear maturation pathways of rat liver ribosomal ribonucleic acids. Biochem J. 1976 Dec 15;160(3):495–503. doi: 10.1042/bj1600495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeva M. D., Dudov K. P., Hadjiolov A. A., Stoykova A. S. Quantitative analysis of rat liver nucleolar and nucleoplasmic ribosomal ribonucleic acids. Biochem J. 1978 May 1;171(2):367–374. doi: 10.1042/bj1710367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabeva M. D., Petrov P. T., Stoykova A. S., Hadjiolov A. A. Contamination of detergent-purified rat liver nuclei by cytoplasmic ribosomes. Exp Cell Res. 1977 Sep;108(2):467–471. doi: 10.1016/s0014-4827(77)80061-x. [DOI] [PubMed] [Google Scholar]
- Dabeva M. D., Todorov B. N., Khadzhiolova A. A. Vnutriiadernoe raspredelenie ribosomnykh RNK iz pecheni krysy. Biokhimiia. 1976 Mar;41(3):458–468. [PubMed] [Google Scholar]
- Dudov K. P., Dabeva M. D., Hadjiolov A. A. Simple agar--urea-gel electrophoretic fractionation of high molecular weight ribonucleic acids. Anal Biochem. 1976 Nov;76(50):250–258. doi: 10.1016/0003-2697(76)90283-9. [DOI] [PubMed] [Google Scholar]
- Eliceiri G. L. Turnover of ribosomal RNA in liver. Biochim Biophys Acta. 1976 Nov 1;447(4):391–394. doi: 10.1016/0005-2787(76)90076-9. [DOI] [PubMed] [Google Scholar]
- Emerson C. P. Regulation of the synthesis and the stability of ribosomal RNA during contact inhibition of growth. Nat New Biol. 1971 Jul 28;232(30):101–106. doi: 10.1038/newbio232101a0. [DOI] [PubMed] [Google Scholar]
- GIRARD M., LATHAM H., PENMAN S., DARNELL J. E. ENTRANCE OF NEWLY FORMED MESSENGER RNA AND RIBOSOMES INTO HELA CELL CYTOPLASM. J Mol Biol. 1965 Feb;11:187–201. doi: 10.1016/s0022-2836(65)80050-x. [DOI] [PubMed] [Google Scholar]
- Goidl J. A., Canaani D., Boublik M., Weissbach H., Dickerman H. Polyanion-induced release of polyribosomes from HeLa cell nuclei. J Biol Chem. 1975 Dec 10;250(23):9198–9205. [PubMed] [Google Scholar]
- Hadjiolov A. A., Milchev G. I. Synthesis and maturation of ribosomal ribonucleic acids in isolated HeLa cell nuclei. A tracer study on the topology of the 45S precursor of ribosomal ribonucleic acids. Biochem J. 1974 Aug;142(2):263–272. doi: 10.1042/bj1420263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiolov A. A., Nikolaev N. Maturation of ribosomal ribonucleic acids and the biogenesis of ribosomes. Prog Biophys Mol Biol. 1976;31(2):95–144. doi: 10.1016/0079-6107(78)90006-8. [DOI] [PubMed] [Google Scholar]
- Hadjiolov A. A. Studies on the turnover and messenger activity of rat-liver ribonucleic acids. Biochim Biophys Acta. 1966 Jun 22;119(3):547–556. doi: 10.1016/0005-2787(66)90131-6. [DOI] [PubMed] [Google Scholar]
- Hogan B. L., Korner A. The role of ribosomal subunits and 80-S monomers in polysome formation in an ascites tumour cell. Biochim Biophys Acta. 1968 Nov 20;169(1):139–149. doi: 10.1016/0005-2787(68)90015-4. [DOI] [PubMed] [Google Scholar]
- Hunt J. A. Ribonucleic acid synthesis in rabbit erythroid cells. Biochem J. 1976 Dec 15;160(3):727–744. doi: 10.1042/bj1600727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joklik W. K., Becker Y. Studies on the genesis of polyribosomes. I. Origin and significance of the subribosomal particles. J Mol Biol. 1965 Sep;13(2):496–510. doi: 10.1016/s0022-2836(65)80112-7. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Hodnett J. L., Steele W. J., Busch H. Synthesis of 28-S RNA in the nucleolus. Biochim Biophys Acta. 1966 Jul 20;123(1):116–125. doi: 10.1016/0005-2787(66)90164-x. [DOI] [PubMed] [Google Scholar]
- Myhill J. Investigation of the effect of data error in the analysis of biological tracer data. Biophys J. 2008 Dec 31;7(6):903–911. doi: 10.1016/S0006-3495(67)86628-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E. Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 1966 Nov 11;154(3750):786–789. doi: 10.1126/science.154.3750.786. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Purtell M. J., Anthony D. D. Changes in ribosomal RNA processing paths in resting and phytohemagglutinin-stimulated guinea pig lymphocytes. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3315–3319. doi: 10.1073/pnas.72.9.3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINER J. M. The study of metabolic turnover rates by means of isotopic tracers. II. Turnover in a simple reaction system. Arch Biochem Biophys. 1953 Sep;46(1):80–99. doi: 10.1016/0003-9861(53)90171-4. [DOI] [PubMed] [Google Scholar]
- Reiner J. M. Recent advances in molecular pathology. Isotopic analysis of metabolic systems. I. Exp Mol Pathol. 1974 Feb;20(1):78–108. doi: 10.1016/0014-4800(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Adams H. R., Smetana K., Busch H. Isolation of the outer layer of the nuclear envelope. Composition of the RNA. Exp Cell Res. 1969 May;55(2):185–197. doi: 10.1016/0014-4827(69)90480-7. [DOI] [PubMed] [Google Scholar]
- Toniolo D., Basilico C. Processing of ribosomal RNA in a temperature sensitive mutant of BHK cells. Biochim Biophys Acta. 1976 Apr 2;425(4):409–418. doi: 10.1016/0005-2787(76)90005-8. [DOI] [PubMed] [Google Scholar]
- Tsurugi K., Morita T., Ogata K. Mode of degradation of ribosomes in regenerating rat liver in vivo. Eur J Biochem. 1974 Jun 1;45(1):119–126. doi: 10.1111/j.1432-1033.1974.tb03536.x. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Warner J. R., Darnell J. E. Ribosomal precursor particles in the HeLa cell nucleus. J Mol Biol. 1967 Apr 28;25(2):235–251. doi: 10.1016/0022-2836(67)90140-4. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Loening U., Willems M., Penman S. Acrylamide gel electrophoresis of HeLa cell nucleolar RNA. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1088–1095. doi: 10.1073/pnas.58.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Processing of 45 s nucleolar RNA. J Mol Biol. 1970 Jan 28;47(2):169–178. doi: 10.1016/0022-2836(70)90337-2. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Penman S. Small molecular weight monodisperse nuclear RNA. J Mol Biol. 1968 Dec;38(3):289–304. doi: 10.1016/0022-2836(68)90387-2. [DOI] [PubMed] [Google Scholar]
- Winicov I. Alternate temporal order in ribosomal RNA maturation. J Mol Biol. 1976 Jan 15;100(2):141–155. doi: 10.1016/s0022-2836(76)80145-3. [DOI] [PubMed] [Google Scholar]
- Wolf S. F., Schlessinger D. Nuclear metabolism of ribosomal RNA in growing, methionine-limited, and ethionine-treated HeLa cells. Biochemistry. 1977 Jun 14;16(12):2783–2791. doi: 10.1021/bi00631a031. [DOI] [PubMed] [Google Scholar]
- ZILVERSMIT D. B. The design and analysis of isotope experiments. Am J Med. 1960 Nov;29:832–848. doi: 10.1016/0002-9343(60)90117-0. [DOI] [PubMed] [Google Scholar]


