Abstract
Purpose
With the growing interest in exploring radiolanthanides for nuclear medicine applications, the question arises as to whether they are generally interchangeable without affecting a biomolecule’s pharmacokinetic properties. The goal of this study was to investigate similarities and differences of four (radio)lanthanides simultaneously applied as complexes of biomolecules or in ionic form.
Methods
Inductively coupled plasma mass spectrometry (ICP-MS) was employed for the simultaneous detection of four lanthanides (Ln = lutetium, terbium, gadolinium and europium) in biological samples. In vitro tumor cell uptake and in vivo biodistribution studies were performed with Ln-DOTATATE, Ln-DOTA-LM3, Ln-PSMA-617 and Ln-OxFol-1. AR42J cells, PC-3 PIP cells and KB cells expressing the somatostatin receptor, the prostate-specific membrane antigen and the folate receptor, respectively, were used in vitro as well as to obtain the respective tumor mouse models for in vivo studies. The distribution of lanthanides in ionic form was investigated in immunocompetent mice. Dual-isotope SPECT/CT imaging studies were performed with mice administered with the radiolabeled biomolecules or chloride salts of lutetium-177 and terbium-161.
Results
Similar in vitro cell uptake was observed for all four lanthanide complexes of each biomolecule into the respective tumor cell lines. AR42J tumor uptake of Ln-DOTATATE and Ln-DOTA-LM3 in mice showed similar values for all lanthanide complexes (3.8‒5.1% ID/g and 4.5‒5.0% ID/g; 1 h p.i., respectively). Accumulation of Ln-PSMA-617 in PC-3 PIP tumors (24–25% ID/g; 1 h p.i.) and of Ln-OxFol-1 in KB tumors (28–31% ID/g; 24 h p.i.) were also equal for the four lanthanide complexes of each biomolecule. After injection of lanthanide chloride salts (LnCl3; Ln = natLu, natTb, natGd, natEu), the liver uptake was different for each metal (~ 12% ID/g, ~ 22% ID/g, ~ 31% ID/g and ~ 37% ID/g; 24 h p.i., respectively) which could be ascribed to the radii of the respective lanthanide ions. In the bones, accumulation was considerably higher for lutetium than for other lanthanides (25 ± 5% ID/g vs. 14‒15% ID/g; 24 h p.i.). These data were confirmed visually by 177Lu/161Tb-based dual-isotope SPECT/CT images.
Conclusions
The presented study confirmed similar properties of Ln-complexes, suggesting that lutetium-177 can be replaced by other radiolanthanides, most probably without affecting the tissue distribution profile of the resultant radiopharmaceuticals. On the other hand, the different radii of the lanthanide ions affected their uptake and resorption mechanisms in liver and bones when injected in uncomplexed form.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00259-024-07018-9.
Keywords: Lanthanides, Lutetium, Terbium, Gadolinium, Europium, Multiplexed ICP-MS analysis, Dual-isotope SPECT imaging, Somatostatin receptor, Folate receptor, Prostate-specific membrane antigen
Introduction
In the field of nuclear oncology, there is growing interest in using radiolanthanides in combination with tumor target-specific biomolecules which makes them the preferred metals for the next-generation radiotheragnostics [1, 2]. The β¯-particle-emitting radiolanthanide, lutetium-177 (T1/2 = 6.65 d, Eβ−average = 134 keV), is the most commonly used therapeutic radionuclide in clinics [3]. More recently, terbium-161, another radiolanthanide with similar decay characteristics (T1/2 = 6.95 d [4]; Eβ−average = 154 keV) and γ-ray emission for SPECT imaging has gained considerable attention in the community [5, 6]. In addition to β¯-particles, terbium-161 also emits short-ranged electrons (conversion and Auger electrons), which are potentially beneficial for the treatment of micrometastases [7–9]. In general, lanthanides like lutetium and terbium exhibit similar chemical properties, thereby enabling their stable coordination with a DOTA chelator as demonstrated by similar logK values of 25.4 and 24.2 for Lu-DOTA and Tb-DOTA, respectively, along with other lanthanides including gadolinium and europium (Gd-DOTA: 24.6 and Eu-DOTA: 23.4) [10–12].
Several preclinical studies confirmed equal tissue distribution profiles of 177Lu- and 161Tb-based radiopharmaceuticals in mice [13–17]. Further exemplary studies focused on other radiolanthanides and showed the similarities of 149Pm- and 166Ho-labeled biotin or 149Pm- and 153Sm-labeled bombesin analogues to the 177Lu-based counterparts [18, 19]. Based on these findings, it can be assumed that any DOTA- or DOTAGA-functionalized biomolecule that is currently used with lutetium-177 could also be employed with terbium-161 and potentially other radiolanthanides without affecting its pharmacokinetic properties [19, 20]. In order to ultimately prove this hypothesis, a head-to-head comparison of several different radiolanthanides would be necessary. In principle, simultaneous measurement of radiolanthanides would, indeed, be feasible based on the characteristic γ-ray emission profile of each radiolanthanide using γ-spectrometry as previously reported [21]. However, a direct comparison of radiolanthanides other than lutetium-177 and terbium-161 is challenging due to their currently scarce availability and the fact that some radiolanthanides decay considerably faster than others [21, 22]. Comparison of lanthanide complexes of stable isotopes is more practical because of their commercial availability and the absence of decay-related factors, such as half-life and intensity of γ-ray emission. Additionally, naturally-occurring lanthanides can be detected at trace levels (ng/L) using inductively coupled plasma mass spectrometry (ICP-MS) [23–27]. Its multiplexing capability allows the quantification of more than one metal isotope at the same time, enabling the simultaneous detection of several lanthanides within the same sample [28–31].
The aim of this study was to employ ICP-MS for a direct comparison of four different lanthanides (Ln = lutetium, terbium, gadolinium or europium). Each biomolecule, namely DOTATATE, DOTA-LM3, PSMA-617 and OxFol-1 [32, 33], was labeled with the four lanthanides and applied as a cocktail for in vitro tumor cell uptake studies as well as for biodistribution studies in tumor-bearing mice. In addition, in vitro and in vivo studies were performed with the four lanthanides applied as chloride salts (LnCl3) or DTPA complexes (Ln-DTPA). Lutetium-177 and terbium-161, the two most relevant radiolanthanides in the context of medical applications, were investigated regarding their in vivo behavior using dual-isotope SPECT/CT imaging after application of the respective mixture of radiometal complexes or radiometal salts.
Materials and methods
Preparation of biomolecules labeled with naturally-occurring, stable lanthanide isotopes
DOTATATE [34], DOTA-LM3 [35], PSMA-617 [36] and OxFol-1 [32, 33, 37, 38] were labeled with the naturally-occurring isotopes of lutetium, terbium, gadolinium and europium (measured and herein indicated as lutetium-175, terbium-159, gadolinium-157 and europium-153, respectively) according to a previously published protocol [25]. natLuCl3, natGdCl3 and natEuCl3 were obtained from Sigma Aldrich Chemistry, Steinheim, Germany; natTbCl3 was obtained from ABCR GmbH, Karlsruhe, Germany. The identity of the products as well as quantitative and stoichiometric labeling of the tumor targeting agents was confirmed by mass determination using ultra performance liquid chromatography-mass spectrometry (UPLC-MS) (Supplementary Material). Solutions containing the Ln-labeled biomolecules were injected into an iron-free ultra performance liquid chromatography system, connected to a triple quadrupole ICP-MS instrument [25] to detect potential traces of uncoordinated metal ions. A chemical purity of ≥ 97% of all lanthanide-labeled tumor targeting agents was confirmed (Supplementary Material, Fig. S1-S3).
ICP-MS analysis of cell and tissue samples
Cells and tissue samples were analyzed after microwave-assisted nitric acid digestion as previously reported [25]. In brief, cell and tissue samples were hydrolyzed using concentrated nitric acid (purified by redistillation, ≥ 99.999%, trace metal basis, Merck KGaA, Darmstadt, Germany). Holmium-165 (50 or 90 µL of a 0.1 mg/L solution in 2% (v/v) nitric acid) was added to each sample for the correction of potential matrix effects and the identification of volume losses during microwave digestion, which was performed at 150 °C for 10 min using a dedicated microwave system (Anton Paar Switzerland AG, Buchs, Switzerland). Samples were diluted using an ESI prepFAST precision M5x dilution system (Elemental Scientific, Inc. Ohama, NE, USA) and analyzed using a triple quadrupole ICP-MS instrument (iCAP TQ, Thermo Fisher, Reinach, Switzerland) as previously reported [25].
In vitro studies
AR42J tumor cells, a somatostatin receptor (SSTR)-positive exocrine rat pancreatic cancer cell line (ECACC 93100618; Health Protection Agency Culture Collections, Salisbury, UK) [39], were cultured in Roswell Park Memorial Institute (RPMI) 1640 medium supplemented with glutamine, antibiotics and 20% fetal calf serum. Prostate-specific membrane antigen (PSMA)-positive PC-3 PIP and PSMA-negative PC-3 flu human prostate cancer cells were obtained from Prof. Dr. Martin Pomper (Johns Hopkins University School of Medicine, Baltimore, MD, USA). These cells were cultured in RPMI 1640 medium supplemented with glutamine, antibiotics, 10% fetal calf serum and puromycin. KB tumor cells, a folate receptor-positive cervical human cancer cell line (ACC-136; German Collection of Microorganisms and Cell cultures, DSMZ, GmbH, Germany) were cultured in RPMI 1640 cell culture medium with glutamine, antibiotics and 10% fetal calf serum, which was replaced at least one week prior to the in vitro studies or tumor cell inoculation by the folate-free analogous medium (FFRPMI, Cell Culture Technologies GmbH, Gravesano, Switzerland) with the respective supplements.
In vitro tumor cell uptake studies
AR42J, PC-3 PIP and KB tumor cells were seeded in 6-well plates and left to grow overnight (Supplementary Material). The following day, a mixture of equal molar amounts of each Ln-DOTATATE (Ln = natLu, natTb, natGd, natEu) at a final concentration of 10 pmol/mL (2.5 pmol/mL per lanthanide complex) was added to each well containing adherent AR42J tumor cells using assay medium. The same procedure was performed with a mixture of the Ln-DOTA-LM3 complexes, the Ln-PSMA-617 complexes and the Ln-OxFol-1 complexes using AR42J, PC-3 PIP and KB tumor cells, respectively.
After a 1-h and 4-h incubation period, the tumor cells were rinsed 3 times with phosphate-buffered saline, pH 7.4 (PBS), to determine the total uptake of the biomolecules. The internalized fraction was assessed by rinsing the cells in addition with an acidic stripping buffer as previously reported [15, 37, 40]. Cells were detached using trypsin and transferred to microwave vials (MG5, 4 mL, Anton Paar Switzerland AG, Buchs, Switzerland) for microwave digestion and subsequent measurement using ICP-MS as described above (Supplementary Material).
A mixture of equal molar amounts of the lanthanide chloride salts (100 nM stock solutions of natLuCl3, natTbCl3, natGdCl3, and natEuCl3, diluted in saline with an adjusted pH value to 3.5‒4.0) was prepared to obtain a final concentration of 10 pmol/mL (2.5 pmol/mL per lanthanide salt) and tested with AR42J, PC-3 PIP and KB tumor cells according to the same protocol as described for the Ln-labeled biomolecules (Supplementary Material). Analogous in vitro cell uptake studies were also performed with lanthanide metal complexes of diethylenetriaminepentaacetic acid (DTPA, Sigma Aldrich Chemie, Steinheim, Germany) to obtain a final concentration of 10 pmol/mL (2.5 pmol/mL per lanthanide complex) (Supplementary Material).
Cell uptake studies were performed in triplicate in 3‒6 independent experiments and the results presented as the average ± standard deviation (SD). Data were normalized to the values measured for the 175Lu-labeled tumor targeting agent or [175Lu]LuCl3, which were set as 100% and zero was defined as 0%. Statistical significance of the normalized data was assessed for the comparison of the values obtained for terbium, gadolinium and europium analogues with those obtained for the lutetium counterparts by applying one-way ANOVA with the Dunnett’s post-test using GraphPad Prism software (version 8). A p-value of < 0.05 was considered statistically significant.
In vivo studies
All animal experiments were performed in accordance with the guidelines of the Swiss Regulations for Animal Welfare following all applicable international, national, and/or institutional guidelines for the care and use of laboratory animals. Ethical approval was obtained from the Cantonal Committee of Animal Experimentation and licenses granted by the responsible cantonal authorities (No 75721 and N° 75668). Female CD1 nude mice, female athymic nude mice and female FVB mice were obtained at the age of 5–6 weeks from Charles River Laboratories (Sulzfeld, Germany). Experiments were performed after an acclimatization period of the mice of at least 7 days. Female CD1 nude mice were subcutaneously inoculated with AR42J or KB tumor cells (5 × 106 cells in 100 µL PBS) on the right shoulder as previously reported [15, 37]. Female athymic nude mice were subcutaneously inoculated with PSMA-positive PC-3 PIP tumor cells (6 × 106 cells in 100 µL Hank’s balanced salt solution (HBSS)) on the right shoulder and PSMA-negative PC-3 flu tumor cells (5 × 106 cells in 100 µL HBSS) on the left shoulder [41]. Immunocompetent FVB mice were used without tumors. Biodistribution and SPECT/CT imaging studies were performed 2‒3 weeks after tumor cell inoculation. Mice with KB tumors were fed with a folate-deficient diet (ssniff Spezialdiäten GmbH, Soest, Germany) while all others received standard rodent chow.
Biodistribution studies of lanthanide complexes and lanthanide salts
A mixture of Ln-labeled (Ln = natLu, natTb, natGd and natEu) tumor-targeting agents (2 nmol in total; 0.5 nmol of each lanthanide complex in 100 µL saline containing 0.05% bovine serum albumin (BSA)) was intravenously injected in corresponding tumor-bearing mice (n = 3‒4 mice per timepoint) [15, 37, 41]. The tissue distribution of the lanthanides was investigated in non-tumor-bearing immunocompetent FVB mice after intravenous injection of a mixture of all four LnCl3 in acidic solution (2 nmol in total, 0.5 nmol of each LnCl3, in 100 µL saline, pH 3.5 to 4.0). Mice were sacrificed after 1 h, 24 h or 96 h (n = 3 per timepoint). Ln-DTPA complexes were mixed as described for the lanthanide salts (2 nmol in total, 0.5 nmol for each Ln-DTPA complex) and diluted in 100 µL saline after adjustment of the pH value to 3.5 to 4.0. The FVB mice (n = 3) were sacrificed 1 h after intravenous injection of the Ln-DTPA complexes. Selected organs and tissues of mice were collected, weighed and processed for ICP-MS analysis as reported above (Supplementary Material) [25].
Biodistribution data were obtained from 3 to 4 mice per timepoint and the results presented as the average ± SD for each group. Data were normalized to the values measured for the Lu-labeled tumor-targeting agent, [175Lu]LuCl3 or [175Lu]Lu-DTPA, which were set as 100% and zero was defined as 0%. Statistical significance of the normalized data was assessed for organs and tissues with lanthanide accumulation > 0.5% ID/g, using one-way ANOVA with the Dunnett’s post-test in GraphPad Prism software (version 8). A p-value of < 0.05 was considered statistically significant.
Radiolabeling of the biomolecules
Radiolabeling of DOTATATE, DOTA-LM3, PSMA-617 and OxFol-1 with lutetium-177 and terbium-161 was performed at a molar activity of 20 MBq/nmol under standard radiolabeling conditions at pH 4.5 as previously reported [13, 15, 17]. The radiolabeled biomolecules were obtained with a radiochemical purity of > 99% and used without further purification for in vivo dual-isotope SPECT/CT imaging studies. A 5-fold molar excess of DTPA relative to the tumor-targeting agent was added to prevent the presence of traces of unreacted or released radiolanthanide. L-ascorbic acid (3 mg) was added to the formulation of the radiolabeled biomolecules to protect them from radiolytic degradation (Supplementary Material).
Dual-isotope SPECT/CT imaging
Dual-isotope SPECT studies with mice were performed as previously reported [15, 17]. Tumor-bearing nude mice (n = 2‒3 for each type of radioconjugate) were injected with 20 MBq (1 nmol) of a mixture of 177Lu- and 161Tb-labeled tumor-targeting agents (each 10 MBq, 0.5 nmol) diluted in saline containing 0.05% BSA. Non-tumor-bearing immunocompetent FVB mice (n = 2) were injected with 20 MBq of a mixture of [177Lu]LuCl3 (10 MBq, 13.8 pmol) and [161Tb]TbCl3 (10 MBq, 14.3 pmol) diluted with saline containing 0.05% BSA with a final pH value of 4.5. Additional mice (n = 2) were injected with colloids of lutetium-177 and terbium-161 obtained by diluting the chloride salts with PBS of pH 7.4 (Supplementary Material). SPECT/CT images were acquired 1 h, 4 h and 24 h after injection of the activity solutions using a dedicated small-animal SPECT/CT scanner (NanoSPECT/CT, Mediso Medical Imaging Systems, Budapest, Hungary) and the Nucline software (version 1.02, Mediso Ltd., Budapest, Hungary). Counts stemming from the γ-lines of lutetium-177 (112.9 keV ± 10% and 208.4 ± 10%) and the γ-lines and x-rays of terbium-161 (47.7 keV ± 10% and 74.6 keV ± 10%) were simultaneously registered using the respective energy windows for each radionuclide. Data were reconstructed based on either the γ-lines of lutetium-177 or the γ-lines and x-rays of terbium-161 to obtain separate distribution profiles for each radioconjugate as previously reported [15].
Results
Cell uptake of lanthanide-labeled tumor-targeting agents
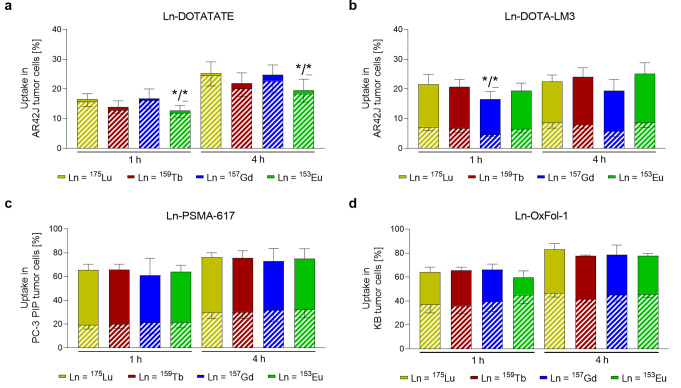
All four lanthanide complexes of each investigated biomolecule (Ln-DOTATATE, Ln-DOTA-LM3, Ln-PSMA-617 and Ln-OxFol-1; measured Ln = 175Lu, 159Tb, 157Gd and 153Eu) demonstrated similar cell uptake and internalization patterns (Fig. 1). Uptake of Ln-DOTATATE and Ln-DOTA-LM3 were within a comparable range of 15‒20% and 20‒25% of the total added amount of complex after a 1-h and 4-h incubation period, respectively, whereas effective internalization was only seen in the case of Ln-DOTATATE (~ 14% and ~ 21%, respectively) (Fig. 1a/b). Uptake of the Ln-PSMA-617 complexes reached 61‒66% (p > 0.05) and 73‒76% (p > 0.05) after a 1-h and 4-h incubation period, respectively, while the internalized fractions reached ~ 20% (p > 0.05) and ~ 30% (p > 0.05) after the same incubation periods (Fig. 1c). This also held true for Ln-OxFol-1 complexes, which showed 60‒66% (p > 0.05) and 78‒83% uptake (p > 0.05) after 1 h and 4 h incubation, respectively, with internalized fractions of ~ 40% (p > 0.05) and ~ 45% (p > 0.05) of total added complex (Fig. 1d).
Fig. 1.
a‒d Total uptake (entire bars) and internalized fractions (hatched part of the bars) of Ln-labeled biomolecules. a/b Ln-DOTATATE and Ln-DOTA-LM3 in AR42J tumor cells; c Ln-PSMA-617 in PC-3 PIP tumor cells and d Ln-OxFol-1 in KB tumor cells. Data (average ± SD, n = 3–6) are presented as percentage of the added Ln-conjugate (set as 100%). Asterisks indicate uptake (*) and internalization (*) values that are significantly different from those measured for the respective 175Lu-labeled analogues (p < 0.05)
Cell uptake of lanthanide chloride salts and lanthanide DTPA complexes
Uptake of the lanthanide salts increased with time, however, it varied between the single lanthanide salts irrespective of the incubation period and tumor cell line (Supplementary Material, Fig. S4). Lanthanide DTPA complexes were neither bound nor internalized by tumor cells as demonstrated by the fact that only background levels of metals (< 20‒60 ppt) were detected in the cell samples (Supplementary Material).
Biodistribution study of tumor-targeting agents labeled with naturally-occurring isotopes
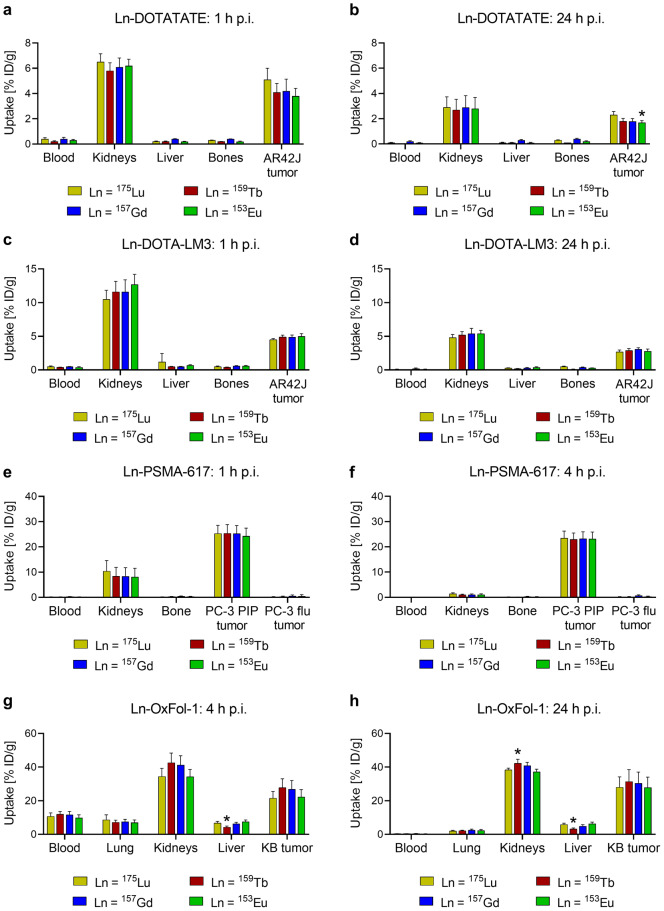
Multiplexed ICP-MS analysis allowed the simultaneous investigation of biomolecules labeled with four naturally-occurring lanthanides in tumor-bearing animals (Supplementary Material, Fig. S5). In AR42J tumor-bearing mice, similar tissue distribution profiles were found for all Ln-DOTATATE complexes. Tumor accumulation was moderate (3.8‒5.1% ID/g at 1 h p.i. and 1.7‒2.3% ID/g at 24 h p.i.) and the uptake in the kidneys only slightly higher (5.8‒6.5% ID/g at 1 h p.i. and 2.7‒2.9% ID/g at 24 h p.i.). No significant differences were observed in the tissue distribution profiles of [175Lu]Lu-DOTATATE and [159Tb]Tb-DOTATATE (p > 0.05) and only minor fluctuations were observed between the data for [153Eu]Eu-DOTATATE and [175Lu]Lu-DOTATATE (Fig. 2a/b). Similar tissue distribution profiles were observed for all Ln-DOTA-LM3 complexes (p > 0.05) with slightly higher tumor uptake (4.5‒5.0% ID/g at 1 h p.i. and 2.7‒3.1% ID/g at 24 h p.i.) than observed for Ln-DOTATATE. Kidney uptake was also equal for all Ln-DOTA-LM3 complexes (11‒13% ID/g at 1 h p.i. and 4.8‒5.4% ID/g at 24 h p.i.) (Fig. 2c/d). Non-specific accumulation in off-target organs and tissues other than the kidneys was low and near the limit of quantification for all Ln-labeled somatostatin analogues (≤ 1% ID/g). Even though significant differences were observed (p < 0.05) in some of the off-target tissue, such as the spleen and the stomach, the absolute values were almost negligible (Supplementary Material, Table S1-S4).
Fig. 2.
a-h Biodistribution data (average ± SD, n = 3–4) obtained at varied timepoints after injection of the mixture of lanthanide complexes (Ln = lutetium, terbium, gadolinium and europium; 0.5 nmol for each biomolecule) a/b DOTATATE and c/d DOTA-LM3 in AR42J tumor-bearing mice (1 h and 24 h p.i.), e/f PSMA-617 in PC-3 PIP and PC-3 flu tumor-bearing mice (1 h and 4 h p.i.) and g/h OxFol-1 in KB tumor-bearing mice (4 h and 24 h p.i.). Tissue uptake is expressed as percent injected dose per gram tissue [% ID/g]. Only organs and tissues relevant for the individual biomolecules are shown. Asterisks indicate uptake (*) values that are significantly different from those measured for the respective 175Lu-labeled biomolecules (p < 0.05)
The four Ln-PSMA-617 complexes demonstrated equal tissue distribution profiles, along with almost identical uptake in PC-3 PIP tumor xenografts (24‒25% ID/g at 1 h p.i. and 23% ID/g at 4 h p.i.; p > 0.05). Uptake in the PSMA-negative PC-3 flu tumor xenografts and normal tissues was low (≤ 1% ID/g) except for the kidneys (8.1‒10% ID/g at 1 h p.i. and 1.0‒1.4% ID/g at 4 h p.i.). As a result, differences in uptake were observed for single timepoints in the stomach and the spleen which were, however, small in absolute values (Fig. 2e/f, Supplementary Material, Table S5-S6).
Ln-OxFol-1 complexes also distributed similarly in KB tumor-bearing mice. Due to the albumin-binding properties of OxFol-1, blood retention was high (9.9‒12% ID/g at 4 h p.i.) and the accumulation in KB tumor xenografts increasing over time (22‒28% ID/g at 4 h p.i. and 28–31% ID/g at 24 h p.i.). Considerable uptake was seen in the lungs (7.0‒8.7% ID/g at 4 h p.i. and 2.0–2.6% ID/g at 24 h p.i.) and in the liver (4.3‒7.5% ID/g at 4 h p.i. and 3.3‒6.4% ID/g at 24 h p.i.). Significant differences (p < 0.05) were found for the liver uptake of [175Lu]Lu-OxFol-1 and [159Tb]Tb-OxFol-1 (6.8 ± 0.9% ID/g vs. 4.3 ± 0.6% ID/g at 4 h p.i. and 5.9 ± 0.7% ID/g vs. 3.3 ± 0.5% ID/g at 24 h p.i.) as well as for the retention in the kidneys (38 ± 1% ID/g vs. 42 ± 2% ID/g at 24 h p.i.) (Fig. 2g/h, Supplementary Material, Table S7-S8).
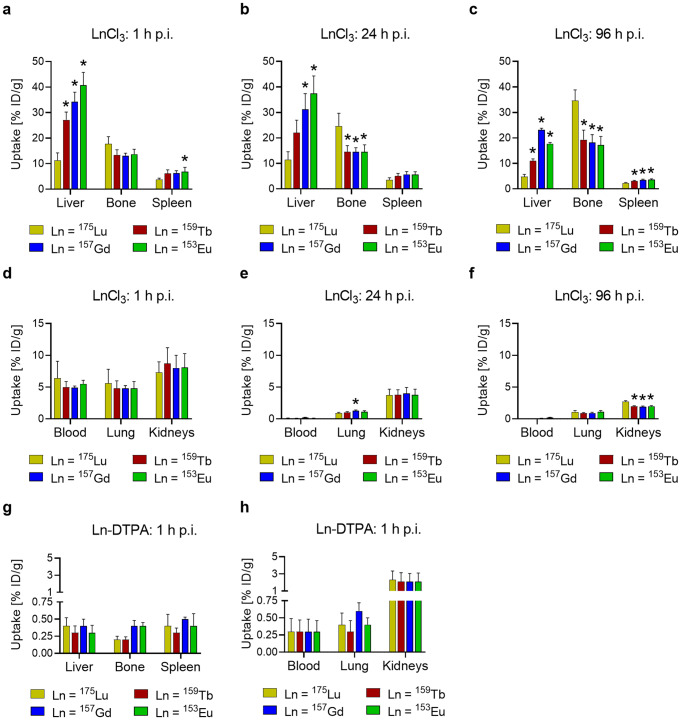
Biodistribution study of lanthanide salts
Application of naturally-occurring lanthanides in their chloride salt form (LnCl3) resulted in uneven distribution profiles. Hepatic accumulation was lowest for lutetium (11 ± 3% ID/g at 1 h p.i. and 12 ± 3% at 24 h p.i.) followed by terbium (27 ± 3% ID/g at 1 h p.i. and 22 ± 5% at 24 h p.i.), gadolinium (34 ± 4% ID/g at 1 h p.i. and 31 ± 6% at 24 h p.i.) and europium (41 ± 5% ID/g at 1 h p.i. and 37 ± 7% at 24 h p.i.) (p < 0.05). The same trend was visible in the spleen, even though the uptake was much lower. Bone accumulation of the lanthanides was increasing over time. At the 96 h p.i. timepoint, the accumulation of lutetium in the bones was about ~ 2-fold higher (35 ± 4% ID/g) than for terbium (19 ± 4% ID/g), gadolinium (17 ± 3% ID/g) and europium (18 ± 3% ID/g) (p < 0.05) (Fig. 3a-c). Also in the kidneys, higher retention was observed after injection of lutetium (2.7 ± 0.2% ID/g at 96 h p.i.) than after application of the other lanthanides (1.9‒2.0% ID/g at 96 h p.i.; p < 0.05). All lanthanides demonstrated equal retention in the blood (4.9‒6.4% ID/g at 1 h p.i.) and the lungs (4.8‒5.6% ID/g at 1 h p.i.) at the early time points and were effectively cleared over time (Fig. 3d-f, Supplementary Material, Tables S9-S11).
Fig. 3.
a‒h Biodistribution data (average ± SD, n = 3) obtained 1 h, 24 h, and 96 h after injection of chloride salts of naturally-occurring lutetium, terbium, gadolinium and europium (0.5 nmol each) in non-tumor-bearing mice. a/b/c Uptake in liver, bone and spleen; d/e/f uptake in blood, lungs and kidneys. Biodistribution data (average ± SD, n = 3) obtained 1 h after injection of DTPA-complexed naturally-occurring lutetium, terbium, gadolinium and europium (0.5 nmol each) in non-tumor-bearing mice. g Uptake in liver, bone and spleen; h uptake in blood, lung and kidneys. Tissue uptake is expressed as percent injected dose per gram tissue [% ID/g]. Asterisks indicate uptake (*) values that are significantly different from those measured for a-f [175Lu]LuCl3 or g/h [175Lu]Lu-DTPA (p < 0.05)
Ln-DTPA complexes were rapidly cleared from the blood circulation and barely detectable in most organs and tissues even as early as 1 h after injection (< 1% ID/g at 1 h p.i.). Only in the kidneys through which the Ln-DTPA complexes were excreted, the lanthanide complexes were still detectable at low concentrations (2.1‒2.3% ID/g at 1 h p.i.; p > 0.05), (Fig. 3g/h, Supplementary Material, Table S12).
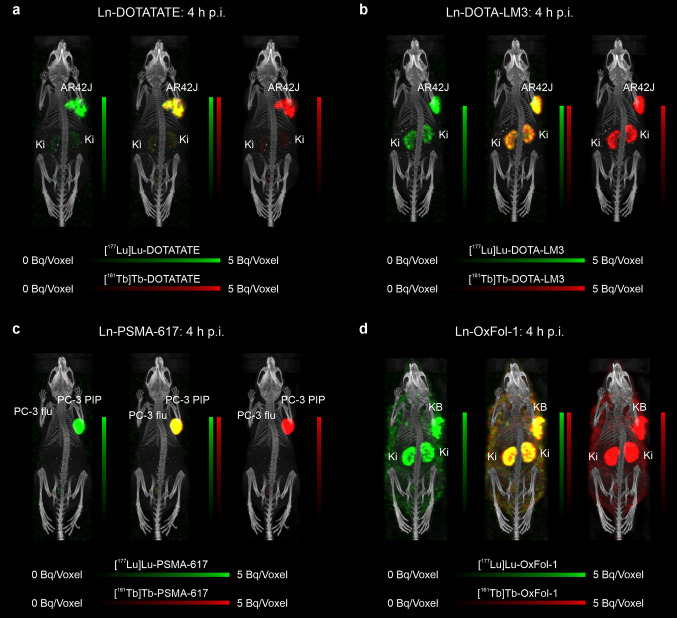
Dual-isotope SPECT/CT imaging
Representative dual-isotope SPECT/CT images of tumor-bearing mice showed nearly identical tissue distribution profiles of the co-administered 177Lu- and 161Tb-labeled biomolecules (Fig. 4, Supplementary Material, Fig. S6-S9). This was not the case upon injection of the radiolanthanide salts, which resulted in an intense uptake and prolonged retention of lutetium in the bones, in particular in the epiphysis. While terbium accumulated less in the bone, it resulted in higher uptake and retention in the liver (Fig. 5, Supplementary Material, Fig. S10). These results are in line with the biodistribution studies that were performed with the respective metal-labeled biomolecules and metal salts of the stable lanthanide isotopes. On the other hand, if the radiolanthanides were administered as colloids in a pH-neutral formulation, an equal accumulation pattern in liver and spleen was seen for lutetium-177 and terbium-161 (Supplementary Material, Fig. S11).
Fig. 4.
a‒d Dual-isotope SPECT/CT images of tumor-bearing mice injected with a mixture of 177Lu-labeled (10 MBq, 0.5 nmol) and 161Tb-labeled (10 MBq, 0.5 nmol) biomolecules at 4 h p.i. a AR42J tumor-bearing mouse injected with Ln-DOTATATE; b AR42J tumor-bearing mouse injected with Ln-DOTA-LM3; c PC-3 PIP/flu tumor-bearing mouse injected with Ln-PSMA-617; d KB tumor-bearing mouse injected with Ln-OxFol-1. (AR42J, SSTR-positive tumor xenograft; PC-3 PIP, PSMA-positive tumor xenograft; PC-3 flu, PSMA-negative tumor xenograft; KB, folate receptor-positive tumor xenograft; Ki, kidney)
Fig. 5.

a‒c Dual-isotope SPECT/CT images of an FVB mouse injected with a mixture of [177Lu]LuCl3 (10 MBq, 13.8 pmol) and [161Tb]TbCl3 (10 MBq, 14.3 pmol) in acidic formulation. a Scan acquired at 1 h p.i.; b scan acquired at 4 h p.i. and c scan acquired at 24 h p.i. (Ki, kidney; Li, liver; Sp, spine; Ep, epiphysis (only indicated in knee joint))
Discussion
This study revealed similar cellular uptake of each Ln-labeled biomolecule irrespective of whether lutetium or any other lanthanide was used. In vivo biodistribution data further confirmed the hypothesis that the tissue distribution profile of a biomolecule was comparable for all investigated lanthanides. Significant differences among the four Ln-labeled versions of each biomolecules were observed in a few cases of non-targeted tissues, in which the uptake was generally low and the measurement of the respective lanthanide close to the detection limit. Such variabilities could potentially be resolved by using larger cohorts of animals in these experiments.
In line with previously performed biodistribution studies using lanthanides applied in salt form at acidic pH [42–46], our study demonstrated preferential accumulation of the lanthanides in the liver and bones of mice. Liver uptake of the herein employed lanthanide chloride salts (Lu: 12% ID/g, Tb: 22% ID/g, Gd: 31% ID/g and Eu: 37% ID/g at 24 h p.i., respectively) correlated positively with their ionic radii (Lu: 0.861 Å, Tb: 0.923 Å, Gd: 0.938 Å and Eu: 0.947 Å [47]) which was in agreement with previously reported data [21]. It is, thus, likely that the hepatic uptake was related to the binding of lanthanide ions to Ca-dependent carriers because of the similarity in ionic radii between lanthanide and calcium ions [47–49]. If the lanthanides were, however, applied in colloid form at neutral pH, they were previously found to accumulate in the liver and spleen as a result of the activity of phagocytic Kupffer cells and splenic macrophages [42, 45].
Furthermore, previous investigations showed that as the ionic radius of the lanthanides decreases and the charge density increases, they exhibit a more favorable exchange with calcium ions in the hydroxyapatite binding sites [50–52]. This may explain the observation of the considerably higher bone accumulation of lutetium compared to the other investigated lanthanides. The observation of liver and bone accumulation of lanthanide ions underlines the relevance of preventing the presence of uncoordinated radiolanthanides in formulations of radiopharmaceuticals, which can be guaranteed by chelation of potential traces of non-reacted or released radiolanthanides [21]. Therefore, the addition of DTPA to radiopharmaceutical formulations has been suggested to capture traces of uncoordinated lutetium-177 [42, 50, 53]. Indeed, our data demonstrated the fast renal excretion of DTPA lanthanide complexes as previously reported for [177Lu]Lu-DTPA [53].
The underlying idea of using other lanthanides as a surrogate for lutetium ‒ based on the assumption that this would not change the properties of the metal complex ‒ has previously been demonstrated by Holtzapfel et al. who used europium instead of lutetium to evaluate PSMA-targeting DOTA conjugates by means of ICP-MS [24]. This suggests the feasibility of using the ICP-MS methodology for the simultaneous screening process of multiple drug candidates labeled with different lanthanides.
Cassette dosing experiments using multiplexed ICP-MS for the detection of multiple elements in biological material could accelerate the drug screening process and eliminate inter-individual differences among the test animals [54]. Importantly, this methodology could significantly reduce the number of test animals, and hence, contribute-AMh substantially to the 3R principle (“Replace, Reduce, Refine”). Despite the high sensitivity of ICP-MS, this methodology may, however, be limited by the relatively large quantities of metal conjugates that are necessary for accurate quantification. This may comprise a risk of receptor saturation in organs with moderate to low receptor expression levels [25]. Moreover, the simultaneous investigation of multiple metal complexes may result in unwanted interactions and affect their distribution profiles [54, 55].
In radiopharmaceutical sciences, the methodology of dual-isotope SPECT imaging is a similar concept which is based on the measurement of γ-ray emission of two different radionuclides (e.g. lutetium-177 and terbium-161). This method can allow the simultaneous investigation of biomolecules labeled with two different radionuclides for longitudinal studies [15, 17]. The present study reinforces the utility of this imaging technology by visualizing the equal in vivo distribution of a mixture and 177Lu- or 161Tb-labeled biomolecules. SPECT images also confirmed the biodistribution data obtained by ICP-MS measurements, demonstrating the increased liver accumulation of terbium-161 but higher bone uptake for lutetium-177 after application of these radiolanthanides in chloride form. When applied as colloids, the accumulation pattern was, however, similar for both radiolanthanides with uptake seen almost exclusively in liver and spleen, in agreement with previous findings of others who applied the lanthanides as colloids at neutral pH [42, 45]. In line with previous studies conducted in rats [34] and dogs [56], our imaging data showed increased localization of the radiolanthanides in the epiphyseal plates as a result of the extensive metal ion resorption. Therefore, apart from revealing the whole-body biodistribution of the radioligands in question over time, dual-isotope SPECT/CT imaging has the advantage of providing information on sub-organ distribution.
Conclusion
Multiplexed ICP-MS analysis allowed simultaneous detection and, hence, direct comparison of co-injected tumor-targeting agents labeled with different lanthanides. While lanthanides in ionic form may have distinct biodistribution patterns due to varying radii and charge densities, only marginal variations arise in their chelator-complexed form. It is, thus, likely that radiolanthanides beyond lutetium-177 and terbium-161 could be interchanged without affecting the biological behavior of the respective radiopharmaceuticals. The comparable behavior of lutetium, terbium, gadolinium and europium in their chelated form opens the possibility of screening drug candidates simultaneously in view of their potential as radiopharmaceuticals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Colin C. Hillhouse, Pascal V. Grundler and Anzhelika Moiseeva for the separation of terbium-161 from target material at PSI. They thank Susan Cohrs, Fan Sozzi-Guo, Sarah D. Busslinger and Darja Beyer for technical assistance with the experiments at PSI, Switzerland. The authors thank Heloïse Hensinger for technical assistance with the experiments at Novartis, Basel, Switzerland.
Funding
Open Access funding provided by Lib4RI – Library for the Research Institutes within the ETH Domain: Eawag, Empa, PSI & WSL.
Ana Katrina Mapanao was funded by a SNSF grant (N° 310030_188978; PI: Cristina Müller); Ana Katrina Mapanao received additional funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement (No 884104). Avni Mehta was funded by the Ulrich Peter & Hans Rudolf Wirz Foundation via Swiss Cancer Research (KFS-5624-08-2022; PI: Cristina Müller). ITM Isotope Technologies Munich GmbH, Germany, provided lutetium-177 for the reported studies.
Data availability
All preclinical data analyzed in this study are included in this published article and in the Supplementary Material. Additional information or more detailed data are available from the corresponding author on reasonable request.
Declarations
Ethical approval
This study was performed in agreement with the national law and PSI-internal guidelines of radiation safety protection. In vivo experiments were approved by the local veterinarian department and ethics committee and conducted in accordance with the Swiss law of animal protection.
Conflict of interest
The University Hospital Basel and the Paul Scherrer Institute have filed a patent with regard to [161Tb]Tb-DOTA-LM3. R. Schibli, N. van der Meulen and C. Müller are listed as co-inventors on the patent application. Merck & Cie, Schaffhausen and PSI have a patent with regard to albumin-binding folate radioconjugates. R. Schibli and C. Müller are listed as co-inventors on the patent application. R. H. Wallimann, R. Kneuer and P. Schindler are employed by Novartis, Basel, Switzerland.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rahel H. Wallimann and Avni Mehta contributed equally to this work.
References
- 1.Teo RD, Termini J, Gray HB. Lanthanides: applications in cancer diagnosis and therapy. J Med Chem. 2016;59:6012–24. 10.1021/acs.jmedchem.5b01975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herlan C, Brase S. Lanthanide conjugates as versatile instruments for therapy and diagnostics. Dalton Trans. 2020;49:2397–402. 10.1039/c9dt04851k. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee S, Pillai MR, Knapp FF. Lutetium-177 therapeutic radiopharmaceuticals: linking chemistry, radiochemistry, and practical applications. Chem Rev. 2015;115:2934–74. 10.1021/cr500171e. [DOI] [PubMed] [Google Scholar]
- 4.Duran MT, Juget F, Nedjadi Y, Bochud F, Grundler PV, Gracheva N, et al. Determination of 161Tb half-life by three measurement methods. Appl Radiat Isot. 2020;159:109085. 10.1016/j.apradiso.2020.109085. [DOI] [PubMed] [Google Scholar]
- 5.Marin I, Ryden T, Van Essen M, Svensson J, Gracheva N, Koster U, et al. Establishment of a clinical SPECT/CT protocol for imaging of 161Tb. EJNMMI Phys. 2020;7:45. 10.1186/s40658-020-00314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lehenberger S, Barkhausen C, Cohrs S, Fischer E, Grünberg J, Hohn A, et al. The low-energy beta- and electron emitter 161Tb as an alternative to 177Lu for targeted radionuclide therapy. Nucl Med Biol. 2011;38:917– 24. 10.1016/j.nucmedbio.2011.02.007. [DOI] [PubMed]
- 7.Champion C, Quinto MA, Morgat C, Zanotti-Fregonara P, Hindie E. Comparison between three promising β¯-emitting radionuclides, 67Cu, 47Sc and 161Tb, with emphasis on doses delivered to minimal residual disease. Theranostics. 2016;6:1611–8. 10.7150/thno.15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hindie E, Zanotti-Fregonara P, Quinto MA, Morgat C, Champion C. Dose deposits from 90Y, 177Lu, 111In, and 161Tb in micrometastases of various sizes: implications for radiopharmaceutical therapy. J Nucl Med. 2016;57:759–64. 10.2967/jnumed.115.170423. [DOI] [PubMed] [Google Scholar]
- 9.Alcocer-Avila ME, Ferreira A, Quinto MA, Morgat C, Hindie E, Champion C. Radiation doses from 161Tb and 177Lu in single tumour cells and micrometastases. EJNMMI Phys. 2020;7:33. 10.1186/s40658-020-00301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma D, Ketring AR, Ehrhardt GJ, Jia W. Production of radiolanthanides and radiotherapy research at MURR. J Radioanal Nucl Chem. 1996;206:119–26. 10.1007/BF02040048. [Google Scholar]
- 11.Bünzli JC. Benefiting from the unique properties of lanthanide ions. Acc Chem Res. 2006;39:53–61. 10.1021/ar0400894. [DOI] [PubMed] [Google Scholar]
- 12.Uusijarvi H, Bernhardt P, Rosch F, Maecke HR, Forssell-Aronsson E. Electron- and positron-emitting radiolanthanides for therapy: aspects of dosimetry and production. J Nucl Med. 2006;47:807–14. [PubMed] [Google Scholar]
- 13.Müller C, Reber J, Haller S, Dorrer H, Bernhardt P, Zhernosekov K, et al. Direct in vitro and in vivo comparison of 161Tb and 177Lu using a tumour-targeting folate conjugate. Eur J Nucl Med Mol Imaging. 2014;41:476–85. 10.1007/s00259-013-2563-z. [DOI] [PubMed] [Google Scholar]
- 14.Müller C, Umbricht CA, Gracheva N, Tschan VJ, Pellegrini G, Bernhardt P, et al. Terbium-161 for PSMA-targeted radionuclide therapy of prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1919–30. 10.1007/s00259-019-04345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borgna F, Barritt P, Grundler PV, Talip Z, Cohrs S, Zeevaart JR, et al. Simultaneous visualization of 161Tb- and 177Lu-labeled somatostatin analogues using dual-isotope SPECT imaging. Pharmaceutics. 2021;13. 10.3390/pharmaceutics13040536. [DOI] [PMC free article] [PubMed]
- 16.Borgna F, Haller S, Rodriguez JMM, Ginj M, Grundler PV, Zeevaart JR, et al. Combination of terbium-161 with somatostatin receptor antagonists-a potential paradigm shift for the treatment of neuroendocrine neoplasms. Eur J Nucl Med Mol Imaging. 2022;49:1113–26. 10.1007/s00259-021-05564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tschan VJ, Busslinger SD, Bernhardt P, Grundler PV, Zeevaart JR, Köster U, et al. Albumin-binding and conventional PSMA ligands in combination with 161Tb: biodistribution, dosimetry, and preclinical therapy. J Nucl Med. 2023;64:1625–31. 10.2967/jnumed.123.265524. [DOI] [PubMed] [Google Scholar]
- 18.Lewis MR, Zhang J, Jia F, Owen NK, Cutler CS, Embree MF, et al. Biological comparison of 149Pm-, 166Ho-, and 177Lu-DOTA-biotin pretargeted by CC49 scfv-streptavidin fusion protein in xenograft-bearing nude mice. Nucl Med Biol. 2004;31:213–23. 10.1016/j.nucmedbio.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Hu F, Cutler CS, Hoffman T, Sieckman G, Volkert WA, Jurisson SS. Pm-149 DOTA bombesin analogs for potential radiotherapy. In vivo comparison with Sm-153 and Lu-177 labeled DO3A-amide-betaAla-BBN(7–14)NH2. Nucl Med Biol. 2002;29:423–30. 10.1016/s0969-8051(02)00290-1. [DOI] [PubMed]
- 20.Schott ME, Schlom J, Siler K, Milenic DE, Eggensperger D, Colcher D, et al. Biodistribution and preclinical radioimmunotherapy studies using radiolanthanide-labeled immunoconjugates. Cancer. 1994;73:993–8. [DOI] [PubMed] [Google Scholar]
- 21.Beyer GJ, Offord R, Kunzi G, Aleksandrova Y, Ravn U, Jahn S, et al. The influence of EDTMP-concentration on the biodistribution of radio-lanthanides and 225Ac in tumor-bearing mice. The ISOLDE collaboration. Nucl Med Biol. 1997;24:367–72. 10.1016/s0969-8051(97)00011-5. [DOI] [PubMed] [Google Scholar]
- 22.Beyer GJ, Miederer M, Vranjes-Duric S, Comor JJ, Kunzi G, Hartley O, et al. Targeted alpha therapy in vivo: direct evidence for single cancer cell kill using 149Tb-rituximab. Eur J Nucl Med Mol Imaging. 2004;31:547–54. 10.1007/s00259-003-1413-9. [DOI] [PubMed] [Google Scholar]
- 23.Boros E, Pinkhasov OR, Caravan P. Metabolite profiling with HPLC-ICP-MS as a tool for in vivo characterization of imaging probes. EJNMMI Radiopharm Chem. 2018;3:2. 10.1186/s41181-017-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holzapfel M, Mutas M, Chandralingam S, von Salisch C, Peric N, Segelke T, et al. Nonradioactive cell assay for the evaluation of modular prostate-specific membrane antigen targeting ligands via inductively coupled plasma mass spectrometry. J Med Chem. 2019;62:10912–8. 10.1021/acs.jmedchem.9b01606. [DOI] [PubMed] [Google Scholar]
- 25.Wallimann RH, Schindler P, Hensinger H, Tschan VJ, Busslinger SD, Kneuer R, et al. Inductively coupled plasma mass spectrometry - a valid method for the characterization of metal conjugates in view of the development of radiopharmaceuticals. Mol Pharm. 2023;20:2150–8. 10.1021/acs.molpharmaceut.2c01092. [DOI] [PubMed] [Google Scholar]
- 26.Wallimann RH, Hensinger H, Müller C, Schibli R, Kneuer R, Schindler P. Liquid chromatography ICP-MS to assess the stability of 175Lu- and natGa-based tumor-targeting agents towards the development of 177Lu- and 68Ga-labeled radiopharmaceuticals. Pharmaceutics. 2024;16. 10.3390/pharmaceutics16030299. [DOI] [PMC free article] [PubMed]
- 27.Schwarz G, Müller L, Beck S, Linscheid MW. DOTA based metal labels for protein quantification: a review. J Anal at Spectrom. 2014;29:221–33. 10.1039/C3JA50277E. [Google Scholar]
- 28.Elias A, Crayton SH, Warden-Rothman R, Tsourkas A. Quantitative comparison of tumor delivery for multiple targeted nanoparticles simultaneously by multiplex ICP-MS. Sci Rep. 2014;4:5840. 10.1038/srep05840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kanje S, Herrmann AJ, Hober S, Müller L. Next generation of labeling reagents for quantitative and multiplexing immunoassays by the use of LA-ICP-MS. Analyst. 2016;141:6374–80. 10.1039/c6an01878e. [DOI] [PubMed] [Google Scholar]
- 30.Buckle T, van der Wal S, van Malderen SJ, Müller L, Kuil J, van Unen V, et al. Hybrid imaging labels: providing the link between mass spectrometry-based molecular pathology and theranostics. Theranostics. 2017;7:624–33. 10.7150/thno.17484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nagayasu M, Takano Y, Ozeki K. Development of a new method to evaluate the biodistribution of antibodies using non-radioactive metal labeling and inductively coupled plasma mass spectrometry. Pharm Res. 2023;40:1807–19. 10.1007/s11095-023-03541-w. [DOI] [PubMed] [Google Scholar]
- 32.Siwowska K, Haller S, Bortoli F, Benešova M, Groehn V, Bernhardt P, et al. Preclinical comparison of albumin-binding radiofolates: impact of linker entities on the in vitro and in vivo properties. Mol Pharm. 2017;14:523–32. 10.1021/acs.molpharmaceut.6b01010. [DOI] [PubMed] [Google Scholar]
- 33.Deberle LM, Benešova M, Becker AE, Ratz M, Guzik P, Schibli R, et al. Novel synthetic strategies enable the efficient development of folate conjugates for cancer radiotheranostics. Bioconjug Chem. 2021;32:1617–28. 10.1021/acs.bioconjchem.1c00198. [DOI] [PubMed] [Google Scholar]
- 34.de Jong M, Breeman WA, Bernard BF, Bakker WH, Schaar M, van Gameren A, et al. [177Lu-DOTA0,Tyr3] octreotate for somatostatin receptor-targeted radionuclide therapy. Int J Cancer. 2001;92:628–33. [DOI] [PubMed] [Google Scholar]
- 35.Fani M, Del Pozzo L, Abiraj K, Mansi R, Tamma ML, Cescato R, et al. PET of somatostatin receptor-positive tumors using 64Cu- and 68Ga-somatostatin antagonists: the chelate makes the difference. J Nucl Med. 2011;52:1110–8. 10.2967/jnumed.111.087999. [DOI] [PubMed] [Google Scholar]
- 36.Benešova M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–20. 10.2967/jnumed.114.147413. [DOI] [PubMed] [Google Scholar]
- 37.Guzik P, Benešova M, Ratz M, Monne Rodriguez JM, Deberle LM, Schibli R, et al. Preclinical evaluation of 5-methyltetrahydrofolate-based radioconjugates-new perspectives for folate receptor-targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2021;48:972–83. 10.1007/s00259-020-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benešova M, Guzik P, Deberle LM, Busslinger SD, Landolt T, Schibli R, et al. Design and evaluation of novel albumin-binding folate radioconjugates: systematic approach of varying the linker entities. Mol Pharm. 2022;19:963–73. 10.1021/acs.molpharmaceut.1c00932. [DOI] [PubMed] [Google Scholar]
- 39.Hofsli E, Thommesen L, Norsett K, Falkmer S, Syversen U, Sandvik A, et al. Expression of chromogranin A and somatostatin receptors in pancreatic AR42J cells. Mol Cell Endocrinol. 2002;194:165–73. 10.1016/s0303-7207(02)00131-4. [DOI] [PubMed] [Google Scholar]
- 40.Umbricht CA, Benešova M, Schmid RM, Türler A, Schibli R, van der Meulen NP, et al. 44Sc-PSMA-617 for radiotheragnostics in tandem with 177Lu-PSMA-617-preclinical investigations in comparison with 68Ga-PSMA-11 and 68Ga-PSMA-617. EJNMMI Res. 2017;7:9. 10.1186/s13550-017-0257-4. [DOI] [PMC free article] [PubMed]
- 41.Benešova M, Umbricht CA, Schibli R, Müller C. Albumin-binding PSMA ligands: optimization of the tissue distribution profile. Mol Pharm. 2018;15:934–46. 10.1021/acs.molpharmaceut.7b00877. [DOI] [PubMed] [Google Scholar]
- 42.Bingham D, Dobrota M. Distribution and excretion of lanthanides: comparison between europium salts and complexes. Biometals. 1994;7:142–8. 10.1007/BF00140484. [DOI] [PubMed] [Google Scholar]
- 43.Tweedle MF, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Invest Radiol. 1995;30:372–80. 10.1097/00004424-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt A, Bernhardt P, Nilsson O, Ahlman H, Kolby L, Schmitt J, et al. Biodistribution and dosimetry of 177Lu-labeled [DOTA0,Tyr3]octreotate in male nude mice with human small cell lung cancer. Cancer Biother Radiopharm. 2003;18:593–9. 10.1089/108497803322287682. [DOI] [PubMed] [Google Scholar]
- 45.Kartamihardja AA, Nakajima T, Kameo S, Koyama H, Tsushima Y. Impact of impaired renal function on gadolinium retention after administration of gadolinium-based contrast agents in a mouse model. Invest Radiol. 2016;51:655–60. 10.1097/RLI.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 46.Cassells I, Ahenkorah S, Burgoyne AR, Van de Voorde M, Deroose CM, Cardinaels T, et al. Radiolabeling of human serum albumin with terbium-161 using mild conditions and evaluation of in vivo stability. Front Med (Lausanne). 2021;8:675122. 10.3389/fmed.2021.675122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikolova V, Kircheva N, Dobrev S, Angelova S, Dudev T. Lanthanides as calcium mimetic species in calcium-signaling/buffering proteins: the effect of lanthanide type on the Ca2+/Ln3+ competition. Int J Mol Sci. 2023;24. 10.3390/ijms24076297. [DOI] [PMC free article] [PubMed]
- 48.Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations. Effect of ionic radius on the rates of ion entry and exit. J Gen Physiol. 1990;95:679–96. 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palasz A, Czekaj P. Toxicological and cytophysiological aspects of lanthanides action. Acta Biochim Pol. 2000;47:1107–14. [PubMed] [Google Scholar]
- 50.Li WP, Ma DS, Higginbotham C, Hoffman T, Ketring AR, Cutler CS, et al. Development of an in vitro model for assessing the in vivo stability of lanthanide chelates. Nucl Med Biol. 2001;28:145–54. 10.1016/s0969-8051(00)00196-7. [DOI] [PubMed] [Google Scholar]
- 51.Cawthray JF, Creagh AL, Haynes CA, Orvig C. Ion exchange in hydroxyapatite with lanthanides. Inorg Chem. 2015;54:1440–5. 10.1021/ic502425e. [DOI] [PubMed] [Google Scholar]
- 52.De Lama-Odria MDC, Valle LJD, Puiggali J. Lanthanides-substituted hydroxyapatite for biomedical applications. Int J Mol Sci. 2023;24. 10.3390/ijms24043446. [DOI] [PMC free article] [PubMed]
- 53.Breeman WA, van der Wansem K, Bernard BF, van Gameren A, Erion JL, Visser TJ, et al. The addition of DTPA to 177Lu-DOTA0,Tyr3]octreotate prior to administration reduces rat skeleton uptake of radioactivity. Eur J Nucl Med Mol Imaging. 2003;30:312–5. 10.1007/s00259-002-1054-4. [DOI] [PubMed] [Google Scholar]
- 54.Smith NF, Raynaud FI, Workman P. The application of cassette dosing for pharmacokinetic screening in small-molecule cancer drug discovery. Mol Cancer Ther. 2007;6:428–40. 10.1158/1535-7163.MCT-06-0324. [DOI] [PubMed] [Google Scholar]
- 55.He K, Qian M, Wong H, Bai SA, He B, Brogdon B, et al. N-in-1 dosing pharmacokinetics in drug discovery: experience, theoretical and practical considerations. J Pharm Sci. 2008;97:2568–80. 10.1002/jps.21196. [DOI] [PubMed] [Google Scholar]
- 56.Jowsey J, Rowland RE, Marshall JH. The deposition of the rare earths in bone. Radiat Res. 1958;8:490–501. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All preclinical data analyzed in this study are included in this published article and in the Supplementary Material. Additional information or more detailed data are available from the corresponding author on reasonable request.