Abstract
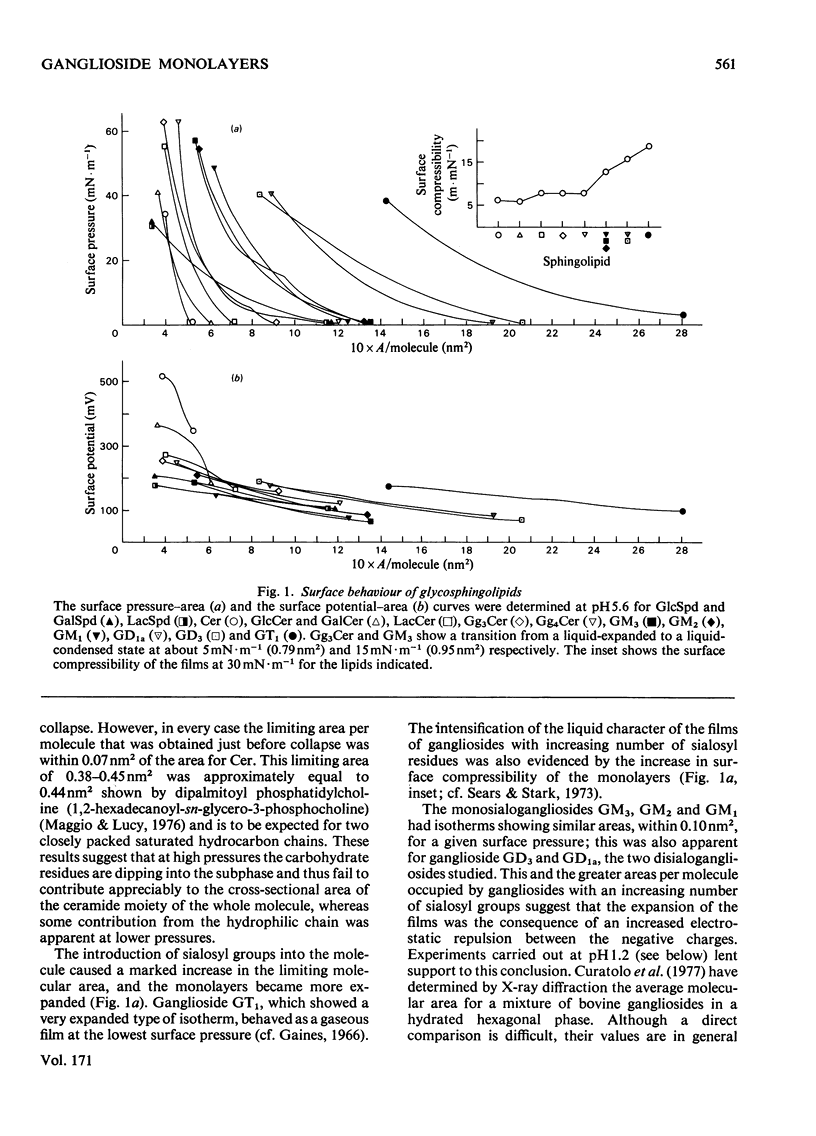
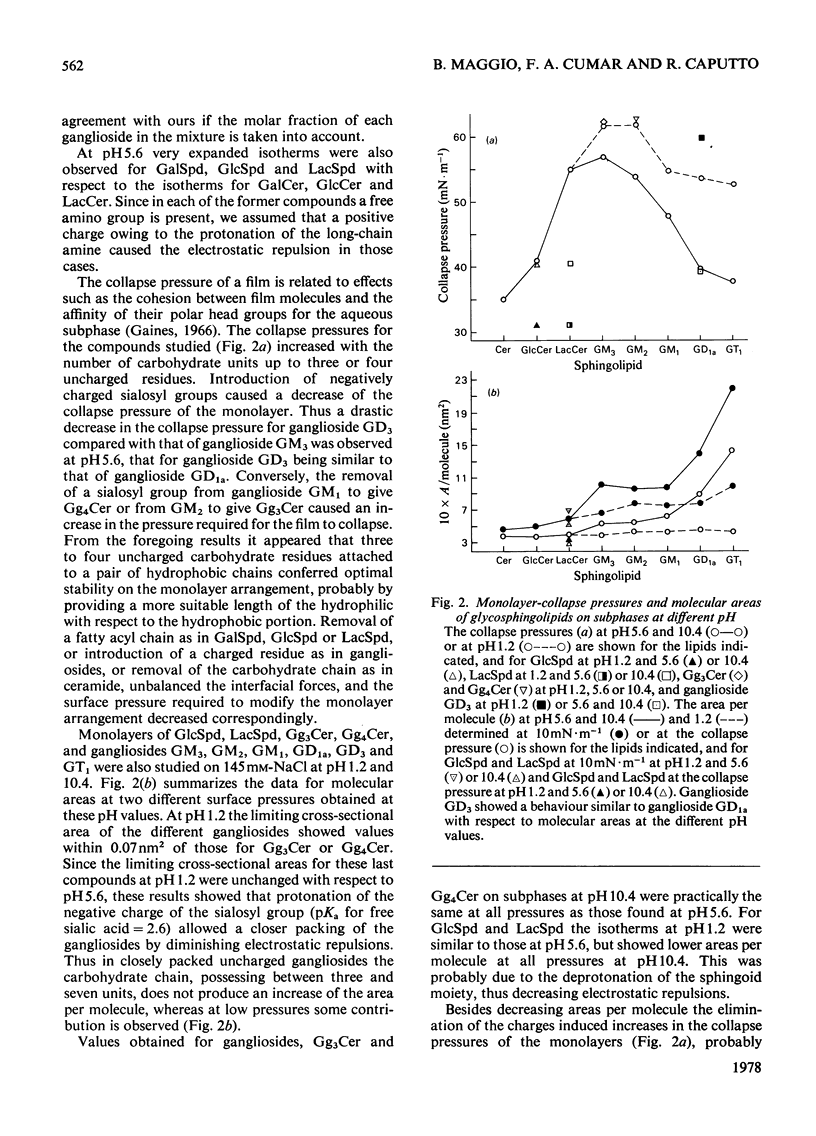
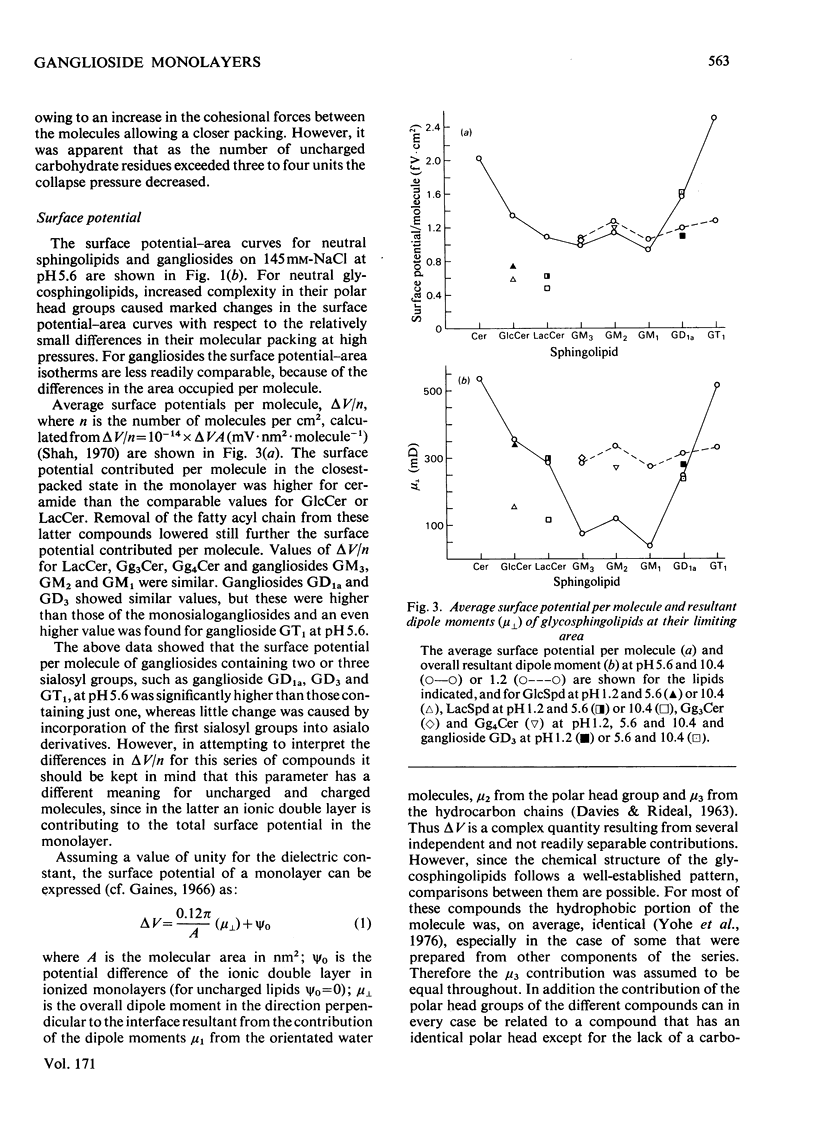
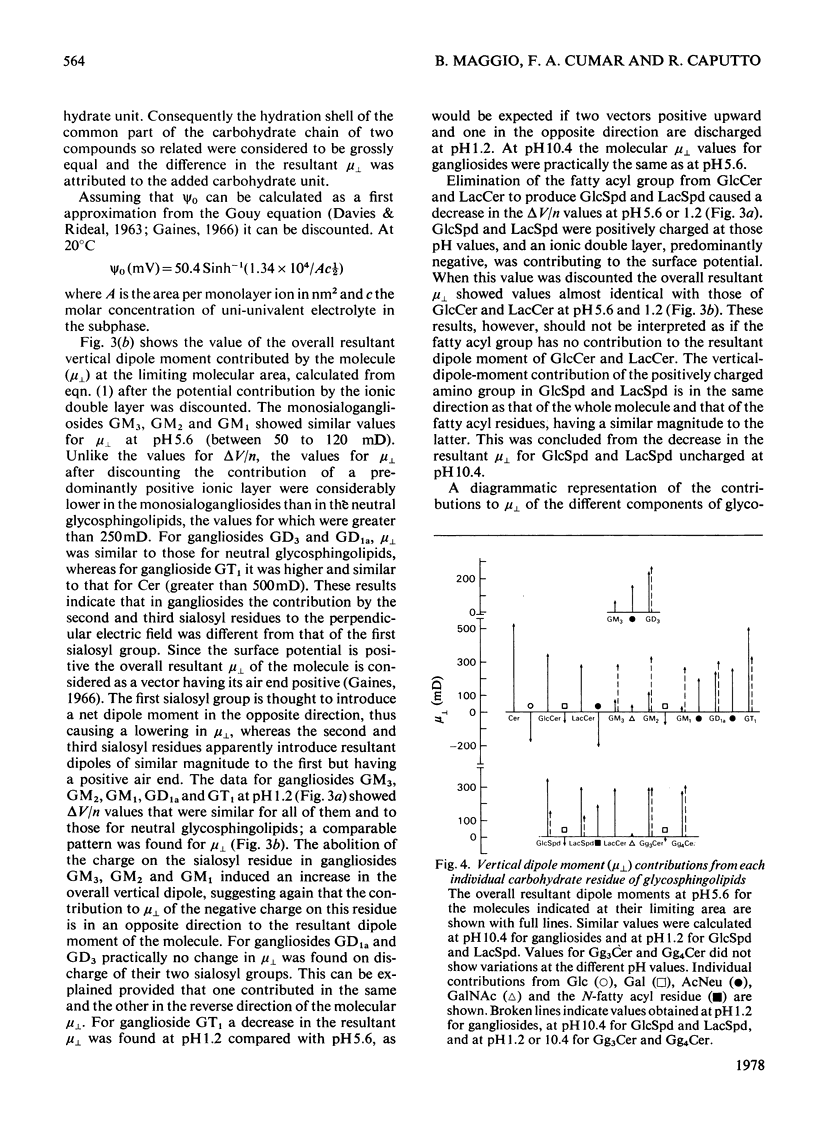
1. The surface behaviour of six different gangliosides and eight chemically related glycosphingolipids was investigated in monolayers at the air-water interface. 2. Mono-, di-, tri and tetra-hexosylceramides had force-area isotherms showing similar limiting molecular areas on 145 mM-NaCl, pH 5.6. The increasing number of negatively charged sialosyl residues in mono-, di- and tri-sialogangliosides induced a progressive increase in the liquid-expanded character of the films and in the limiting area occupied per molecule, owing to electrostatic repulsions. When the ganglioside monolayers were spread on subphases at pH 1.2, the limiting area per molecule was similar to that found for neutral glycosphingolipids. 3. The monolayer collapse pressure at pH 5.6 increased with the number of uncharged carbohydrate units up to when the polar head group contained 3-4 residues. For gangliosides the collapse pressures were lower and decreased from mono- to tri-sialogangliosides. Ganglioside monolayers on subphases at pH 1.2 showed increases in their collapse pressure. 4. The glycosphingolipid monolayers studied had various surface in their collapse pressure. 4. The glycosphingolipid monolayers studied had various surface potentials according to the complexity of the polar head group of the lipid. Attempts to calculate the dipolar contributions to the surface potential from each carbohydrate residue suggest that the second and third sialosyl residues in di- and tri-sialogangliosides contributed with a vertical dipole moment opposite to that of the first sialosyl residue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cumar F. A., Barra H. S., Maccioni H. J., Caputto R. Sulfation of glycosphingolipids and related carbohydrates by brain preparations from young rats. J Biol Chem. 1968 Jul 25;243(14):3807–3816. [PubMed] [Google Scholar]
- Cumar F. A., Fishman P. H., Brady R. O. Analogous reactions for the biosynthesis of monosialo- and disialo-gangliosides in brain. J Biol Chem. 1971 Aug 25;246(16):5075–5084. [PubMed] [Google Scholar]
- Curatolo W., Small D. M., Shipley G. G. Phase behavior and structural characteristics of hydrated bovine brain gangliosides. Biochim Biophys Acta. 1977 Jul 4;468(1):11–20. doi: 10.1016/0005-2736(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Grollman E. F., Lee G., Ambesi-Impiombato F. S., Meldolesi M. F., Aloj S. M., Coon H. G., Kaback H. R., Kohn L. D. Effects of thyrotropin on the thyroid cell membrane: hyperpolarization induced by hormone-receptor interaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2352–2356. doi: 10.1073/pnas.74.6.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPACZYK K. C., RADIN N. S. IN VIVO CONVERSIONS OF CEREBROSIDE AND CERAMIDE IN RAT BRAIN. J Lipid Res. 1965 Jan;6:140–145. [PubMed] [Google Scholar]
- Maccioni H. J., Arce A., Landa C., Caputto R. Rat brain microsomal gangliosides. Accessibility to a neuraminidase preparation and the possible existence of different pools in relation to their biosynthesis. Biochem J. 1974 Feb;138(2):291–298. doi: 10.1042/bj1380291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Polar-group behaviour in mixed monolayers of phospholipids and fusogenic lipids. Biochem J. 1976 May 1;155(2):353–364. doi: 10.1042/bj1550353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Lucy J. A. Studies on mixed monolayers of phospholipids and fusogenic lipids. Biochem J. 1975 Sep;149(3):597–608. doi: 10.1042/bj1490597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Mestrallet M. G., Cumar F. A., Caputto R. Glucose release from liposomes containing gangliosides or other membrane lipids induced by biogenic amines and myelin basic protein. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1265–1272. doi: 10.1016/s0006-291x(77)80116-2. [DOI] [PubMed] [Google Scholar]
- Mestrallet M. G., Cumar F. A., Caputto R. Trisialoganglioside synthesis by a chicken brain sialyltransferase. Comparative study with the similar reaction for the synthesis of disialoganglioside. Mol Cell Biochem. 1977 Jul 5;16(2):63–70. doi: 10.1007/BF01732045. [DOI] [PubMed] [Google Scholar]
- Oldani D., Hauser H., Nichols B. W., Phillips M. C. Monolayer characteristics of some glycolipids at the air-water interface. Biochim Biophys Acta. 1975 Feb 28;382(1):1–9. doi: 10.1016/0005-2736(75)90366-1. [DOI] [PubMed] [Google Scholar]
- Quinn P. J., Sherman W. R. Monolayer characteristics and calcium adsorption to cerebroside and cerebroside sulphate oriented at the air-water interface. Biochim Biophys Acta. 1971 Jun 1;233(3):734–752. doi: 10.1016/0005-2736(71)90173-8. [DOI] [PubMed] [Google Scholar]
- SVENNERHOLM L. CHROMATOGRAPHIC SEPARATION OF HUMAN BRAIN GANGLIOSIDES. J Neurochem. 1963 Sep;10:613–623. doi: 10.1111/j.1471-4159.1963.tb08933.x. [DOI] [PubMed] [Google Scholar]
- Yohe H. C., Roark D. E., Rosenberg A. C20-sphingosine as a determining factor in aggregation of gangliosides. J Biol Chem. 1976 Nov 25;251(22):7083–7087. [PubMed] [Google Scholar]


