Abstract
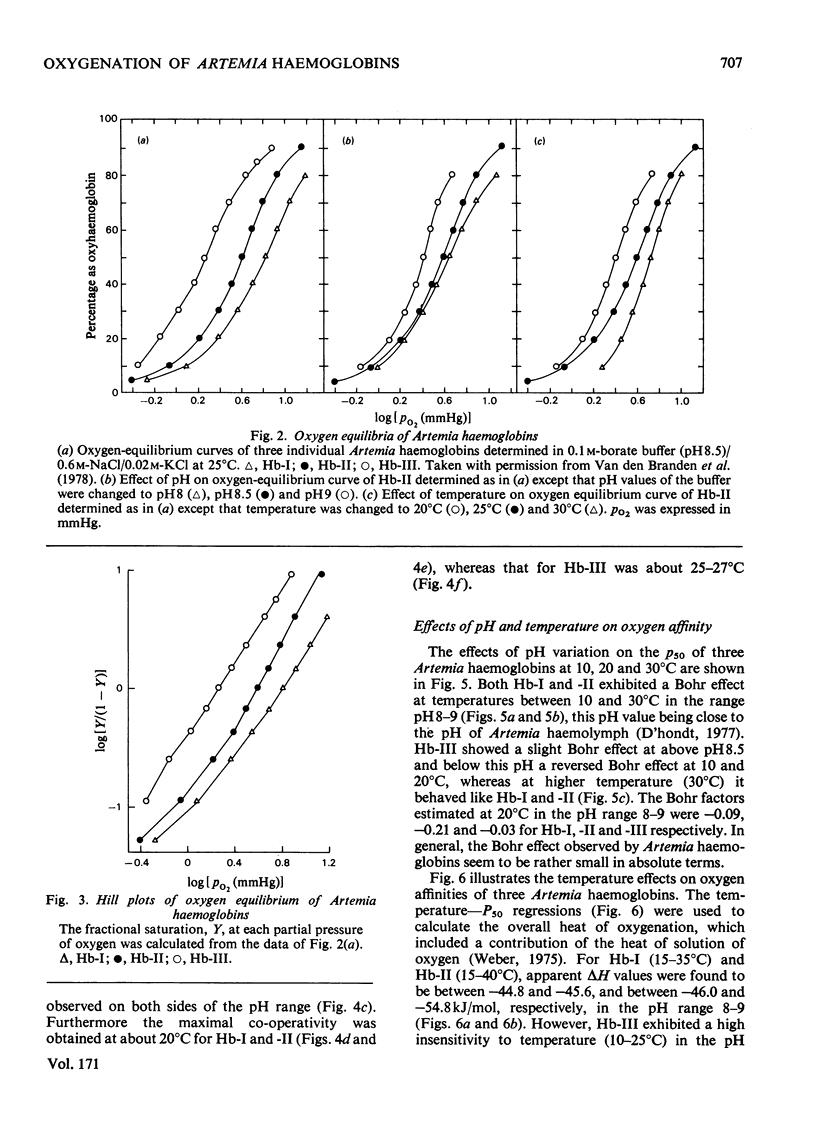
The oxygen-binding characteristics of the three extracellular haemoglobins of brine shrimp (Artemia salina) were studied in vitro by using highly purified preparations. Haemoglobin I is induced last in the development of brine shrimps when functional gills are formed. It has the lowest oxygen affinity (p50 5.34mmHg), an intermediate Bohr effect (ø −0.09 at 20°C) above pH8 and a temperature-sensitivity (ΔH −44.8 to −45.6kJ/mol at pH8–9) comparable with those observed with other invertebrate haemoglobins [Weber & Heidemann (1977) Comp. Biochem. Physiol. A 57, 151–155]. Haemoglobin II, which is the first to be induced, soon after hatching of nauplius larvae, persists generally throughout the whole adult life. It has an intermediate oxygen affinity (p50 3.7mmHg), the highest Bohr effect (ø −0.21 at 20°C) above pH8 and a similar temperature-sensitivity (ΔH −46.0 to −54.8kJ/mol at pH8–9) as haemoglobin I. However, haemoglobin III, which is induced second several hours after the induction of haemoglobin II but disappearing from the haemolymph in the middle of adult life, has the highest oxygen affinity (p50 1.8mmHg), the lowest Bohr effect (ø −0.03 at 20°C) above pH8.5 and a high resistance against temperature variation between 10 and 25°C at pH8.5–9 (ΔH −22.6 to −23.0kJ/mol). At pH7.5–8, haemoglobin III exhibits a similar temperature-sensitivity under 30°C as do other haemoglobins. All three haemoglobins have a rather low co-operativity, with Hill coefficients (h 1.6–1.9 at pH8.5), which are dependent on both pH and temperature. The highest co-operativity was observed at 20°C and pH9 for haemoglobins I and II, whereas it was at 27°C and pH8.5 for haemoglobin III. Thus the oxygen-binding behaviour of haemoglobin III in vitro is significantly different from those of haemoglobins I and II and indicates possibly its specific physiological role in vivo in the adaptive process in the natural environment.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Bannister W. H., Anastasi A. Isolation, characterization and oxygen equilibrium of an extracellular haemoglobin from Eunice aphroditois (Passas). Biochem J. 1976 Oct 1;159(1):35–42. doi: 10.1042/bj1590035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen S. T., Lebherz H. G., Poon M. C., Chow V. H., Grigliatti T. A. The hemoglobins of Artemia salina. I. Determination of phenotype by genotype and environment. Comp Biochem Physiol. 1969 Dec 1;31(5):733–747. doi: 10.1016/0010-406x(69)92073-8. [DOI] [PubMed] [Google Scholar]
- Economides A. P., Wells R. M. The respiratory function of the blood of Neanthes (equal Nereis) virens (Sars) (Polychaeta: Nereidae). Comp Biochem Physiol A Comp Physiol. 1975 May 1;51(1A):219–223. doi: 10.1016/0300-9629(75)90439-9. [DOI] [PubMed] [Google Scholar]
- Heip J., Moens L., Kondo M. Ontogenity of haemoglobins in the brine shrimp, Artemia salina (L.) [proceedings]. Arch Int Physiol Biochim. 1977 Feb;85(1):177–178. [PubMed] [Google Scholar]
- Moens L., Kondo M. Characterization of the extracellular haemoglobins of Artemia salina. Biochem J. 1977 Jul 1;165(1):111–119. doi: 10.1042/bj1650111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens L., Kondo M. Evidence for a dimeric form of Artemia salina extracellular hemoglobins with high-molecular-weight subunits. Eur J Biochem. 1978 Jan 2;82(1):65–72. doi: 10.1111/j.1432-1033.1978.tb11997.x. [DOI] [PubMed] [Google Scholar]
- Moens L., Kondo M. The structure of Artemia salina haemoglobins. A comparative characterisation of four naupliar and adult heamoglobins. Eur J Biochem. 1976 Aug 16;67(2):397–402. doi: 10.1111/j.1432-1033.1976.tb10704.x. [DOI] [PubMed] [Google Scholar]
- Pearson J. S., Wood E. J. Oxygen binding properties of Colus gracilis haemocyanin and its subunits. FEBS Lett. 1974 Nov 15;48(2):246–249. doi: 10.1016/0014-5793(74)80478-3. [DOI] [PubMed] [Google Scholar]
- Sick H., Gersonde K. Method for continuous registration of O2-binding curves of hemoproteins by means of a diffusion chamber. Anal Biochem. 1969 Dec;32(3):362–376. doi: 10.1016/s0003-2697(69)80002-3. [DOI] [PubMed] [Google Scholar]
- Wood E. J., Dalgleish D. G. Murex trunculus haemocyanin. 2. The oxygenation reaction and circular dichroism. Eur J Biochem. 1973 Jun 15;35(3):421–427. doi: 10.1111/j.1432-1033.1973.tb02854.x. [DOI] [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J. Physicochemical properties of Planorbis corneus erythrocruorin. Biochem J. 1975 Aug;149(2):437–445. doi: 10.1042/bj1490437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E. J., Mosby L. J., Robinson M. S. Characterization of the extracellular haemoglobin of Haemopsis sanguisuga (L.). Biochem J. 1976 Mar 1;153(3):589–596. doi: 10.1042/bj1530589. [DOI] [PMC free article] [PubMed] [Google Scholar]



