Abstract
Bottlebrush (BB) polymers, with their densely grafted side chains and unique architecture, are highly advantageous for drug delivery due to their high functional group density for drug conjugation, unimolecular nature, and enhanced biodistribution properties. These attributes enable extended blood circulation half-life, improved tumor tissue penetration, and high tumoral drug accumulation. However, the typically nondegradable, all-carbon backbones of most BB polymers limit their suitability for applications requiring controlled clearance and biodegradability. To address this, we developed degradable BB polymers with poly(disulfide) backbones synthesized via reversible addition–fragmentation chain transfer (RAFT) copolymerization of α-lipoic acid (LA), a renewable and readily available compound, with acrylate-based inimers. These copolymers feature degradable backbones and initiating sites for subsequent BB synthesis. Using an atom transfer radical polymerization (ATRP) grafting-from methodology, we synthesized BB polymers with relatively low dispersities (Đ = 1.30–1.53), high backbone degrees of polymerization (DPbb), and high molar masses (Mn,MALS = 650–2700 kg/mol). The easily cleavable disulfide bonds enabled backbone degradation under mild reducing conditions. Beyond hydrophilic BB with tri(ethylene glycol) methyl ether acrylate (TEGA) side chains, we synthesized BB with cationic, anionic, and zwitterionic side chains, demonstrating broad monomer compatibility. This scalable approach produces water-soluble, degradable BB polymers with tunable architectures and predictable molecular weights. By addressing the need for degradability in BB polymers, this work advances their potential for drug delivery, offering enhanced functionality, biocompatibility, and sustainability.
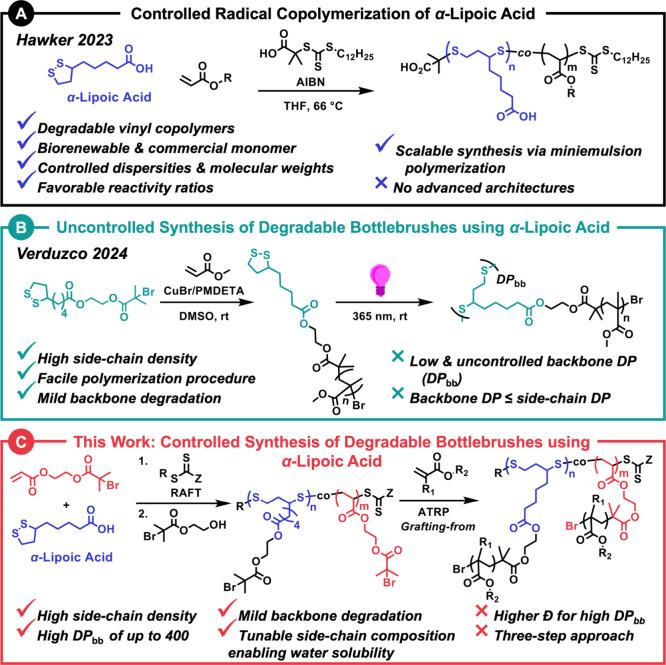
Bottlebrush (BB) polymers consist of a polymer backbone with densely grafted polymer side chains that lead to the elongated bottlebrush structure.1−4 The steric repulsions and relatively short side chains give rise to several unique properties compared to other macromolecular architectures, namely low elastic moduli, high entanglement molecular weights, and tunable self-assembly behavior.5−10 In addition, biomedical applications of BB polymers have been investigated, as they exhibit good stability in solution, long blood circulation, effective tissue penetration, and the ability to carry many therapeutics per macromolecule if used for drug delivery.11−16 If the BBs were made biodegradable, this would further enhance their biocompatibility and ensure the clearance of the BB from the body after achieving their desired effect. Recently, there have been several reports on the synthesis of degradable BB polymers,17−20 with synthetic strategies commonly involving the incorporation of degradable repeat units into the BB main chain. These degradable units include silyl ethers,17,18 thioesters,19 and disulfides,20 which are labile in response to certain conditions or stimuli. However, there are several limitations to the degradable BBs reported thus far, most notably in achieving degradation under mild physiological conditions,17 low side chain density,17−19 low and uncontrolled degrees of polymerization (DP) of the backbone (DPbb) and low DP of the side chains (DPsc),19,20 tedious synthetic procedures,17,19 or limited versatility in side chain functionality.18,20 Notably, You et al. attempted to address these issues in a recent report on a highly controlled living ring-opening metathesis polymerization system that was extended to BBs, incorporating easily degradable 7-oxa-2,3-diaza-norbornenes into backbones.18 However, the BBs presented in this work still did not possess high DPbb, had low polymeric side chain density, and only one example of side chain composition (poly(ethylene glycol) methyl ether). Addressing all these issues enables the facile synthesis of BBs with precise architectures and controlled molecular weights while ensuring their degradability under mild conditions necessary for biocompatibility.
BBs with polymer backbones containing repeating disulfide units, or poly(disulfide)s, are particularly attractive for biological applications such as targeted anticancer drug delivery.23,24 Disulfide bonds are biodegradable and can undergo rapid thiol–disulfide exchange with glutathione (GSH), a physiological antioxidant present at especially high concentrations in tumor cells.25−28 A viable and frequently utilized approach to prepare poly(disulfide)s is through the ring-opening polymerization (ROP) of 1,2-dithiolane monomers.29,30 α-Lipoic acid (LA), a biologically derived and commercially available small molecule containing 1,2-dithiolane moiety, can undergo radical ROP to yield poly(disulfide)s.31−33 Recently, Hawker et al. reported the controlled synthesis of poly(disulfide)s through reversible addition–fragmentation chain transfer (RAFT) copolymerization of LA with acrylates (Figure 1A).21,22 The copolymerization of LA and n-butyl acrylate (BA) yielded polymers with controlled molecular weights and low dispersity (Đ). A kinetic study of the copolymerization revealed faster incorporation of LA compared to BA, favoring the formation of disulfide units in the copolymers. In addition, at 20 and 30% molar feed ratios of LA to BA, the final LA incorporation in the copolymers was 22 and 36%, respectively, which led to favorable degradation profiles of the copolymers with the mild disulfide-reducing agent tris(2-carboxyethyl) phosphine (TCEP). Verduzco et al. reported a grafting-through approach to synthesize degradable BBs with poly(disulfide) backbones by polymerizing an LA-based macromonomer through uncontrolled radical photopolymerization (Figure 1B).20 However, the resulting BBs exhibited uncontrolled molecular weights and small backbone DPs (DPbb ≤ 35). Additionally, the backbone DPs were shorter than those of the grafted side chains (DPsc), resulting in architectures that resembled star-like polymers rather than typical BB polymers, which likely restricts the favorable properties associated with true BB polymers (Figure 1B).
Figure 1.
(A) RAFT copolymerization of LA and various acrylates to obtain degradable vinyl copolymers with controlled molecular weights and dispersities.21,22 (B) Grafting-through approach using light-mediated ROP of LA-functionalized poly(methyl methacrylate) macromonomers to synthesize degradable BB with disulfide-containing backbones.20 (C) In this work, degradable BB polymers were synthesized via ATRP grafting-from macroinitiators prepared through RAFT copolymerization of LA and BiBOEA inimer, followed by postpolymerization modification with HOBiB.
Inspired by this work, we developed a new synthetic strategy to prepare degradable BBs from LA with controlled molecular weights and low Đ, along with tunable chemical structure of side chains affording water solubility for potential use in biomedical applications (Figure 1C). Our approach begins with the RAFT copolymerization of LA and an acrylate-based initiator-monomer (inimer) 2-(2-(bromoisobutyryl)oxy)ethyl acrylate (BiBOEA), resulting in macroinitiator prepolymers (P(LA-co-BiBOEA)).34 They contain pendent α-bromoisobutyrate (BiB) initiating moieties for subsequent atom transfer radical polymerization (ATRP) grafting-from to obtain well-defined BB structures.35,36 We first investigated the kinetics and control of the RAFT copolymerization of LA and BiBOEA at a target DP of 200 and 25 mol % LA feed (Figure 2A). The polymerization was carried out in THF at 70 °C using S-dodecyl-S′-(α,α′-dimethyl-α′′-acetic acid)trithiocarbonate (DDMAT) as the chain transfer agent and azobis(isobutyronitrile) (AIBN) as the radical initiator.
Figure 2.

(A) Reaction scheme for the RAFT copolymerization of LA and BiBOEA. (B) Evolution of SEC traces over the course of the RAFT copolymerization of LA and BiBOEA showing time points taken between 0 and 120 min. (C) Pseudo-first order kinetic plot for conversions of both LA and BiBOEA during the RAFT copolymerization. (D) Plot of Mn,SEC versus total monomer conversion showing good agreement with Mn,theory while maintaining relatively controlled Đ. (E) SEC traces of P(LA-co-BiBOEA) for varying target DPs of 200–1000 and obtained DPs of 100–430.
Size exclusion chromatography with multiangle light scattering (SEC-MALS) analysis showed clean shifts of the SEC traces to lower elution times as the polymer chains grew uniformly during RAFT copolymerization (Figure 2B). The linear pseudo-first-order kinetic plot indicated constant radical concentration throughout the copolymerization and a faster incorporation rate of LA relative to BiBOEA consistent with the findings reported by Hawker et al. (Figure 2C). Moreover, apparent number-average molecular weight (Mn,app) as determined by SEC showed a linear increase with conversion, aligning relatively well with the theoretical number-average molecular weight for quantitative initiation and monomer conversion, determined by 1H NMR spectroscopy (Mn,theory) (Figure 2D). These results collectively demonstrated that RAFT copolymerization of LA and BiBOEA proceeded in a relatively controlled manner. The copolymerization was investigated for different target DPs (200, 300, 500, 700, 1000), and at 20 or 25 mol % feed of LA to ensure the incorporation of ample disulfide units in the backbones for degradation of the resulting BBs (Figure 2E). For all target DPs, after 3 h, BiBOEA conversion reached moderate values of 38–48% as measured by 1H NMR spectroscopy, while LA conversion reached >55%. LA incorporation in the purified polymers ranged from 29 to 35% (Table 1). For reaction times longer than 4 h, both comonomers reached slightly higher conversion, however an increase in Đ was observed. While dispersity remained relatively controlled (Đ = 1.36–1.51) for lower target backbone lengths (DP ≤ 500), it broadened for higher target DPs of 700 and 1000 (Đ = 1.94, 2.25), possibly due to some transfer to the polymer backbone. Nonetheless, SEC of the purified P(LA-co-BiBOEA) copolymers with varying DPs (100, 140, 220, 320, 430) revealed monomodal distributions for all copolymerizations, demonstrating predictable molecular weights and enabling the construction of backbones with higher DPbb compared to previous reports.17−20
Table 1. Characterization of P(LA-co-BiBOEA) Polymers.
| entry | time (h) | LA (equiv) | BiBOEA (equiv) | LA conv.a (%) | BiBOEA conv.a (%) | LA incorp.a (%) | Mn,theoryb (kg/mol) | Mn,appc (kg/mol) | Đc |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 50 | 150 | 66 | 48 | 33 | 26.2 | 22.8 | 1.41 |
| 2 | 3 | 75 | 225 | 59 | 44 | 29 | 34.3 | 30.7 | 1.36 |
| 3 | 3 | 100 | 400 | 55 | 41 | 30 | 55.2 | 40.5 | 1.51 |
| 4 | 3 | 175 | 525 | 59 | 41 | 34 | 78.6 | 53.6 | 1.94 |
| 5 | 3 | 250 | 750 | 55 | 38 | 35 | 104 | 59.9 | 2.25 |
Monomer conversion and LA incorporation were determined by 1H NMR spectroscopy.
Theoretical molar masses (Mn,theory) were determined from the monomer conversion from 1H NMR spectroscopy.
Relative number-average molar masses (Mn,app) and dispersity (Đ) were determined by SEC analysis (THF as eluent) calibrated to poly(methyl methacrylate) standards.
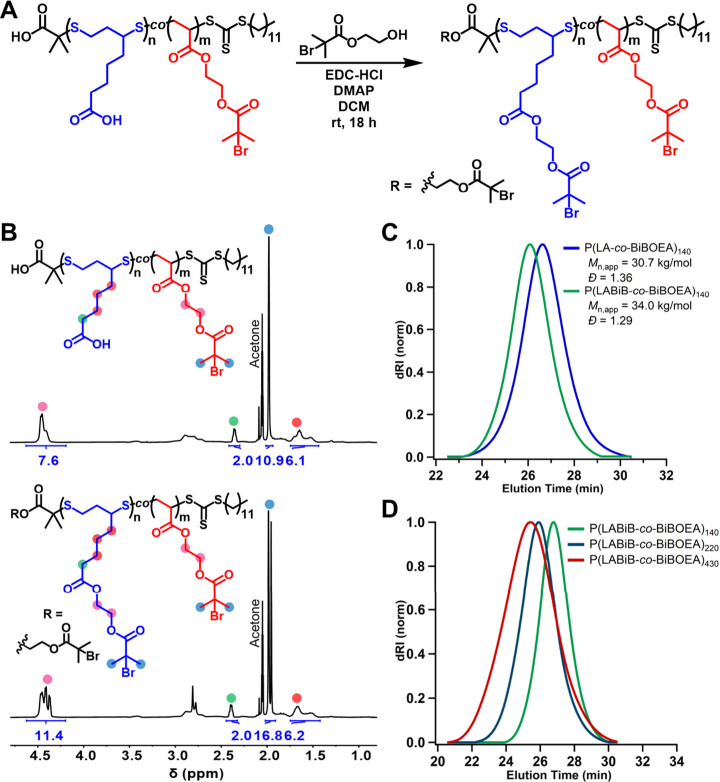
To increase the density of ATRP initiator pendant groups on the macroinitiators and, by extension the side chain density of the BBs, we sought to install additional BiB units on the carboxylic acid pendant groups of LA (Figure 3A). Initially, a RAFT copolymerization of BiBOEA was attempted with an LA inimer containing a BiB moiety (Figure S1). This copolymerization yielded inconsistent results, proceeding to low conversions and multimodal molecular weight distributions (Figure S1). Therefore, the pendant carboxylic acids of the prepolymers were modified postpolymerization with 2-(hydroxyethyl) 2-bromoisobutyrate (HOBiB). From 1H NMR spectroscopy, the modification reached quantitative conversion (Figure 3B), and an increase in molecular weight was observed from SEC consistent with the addition of the BiB units (Figure 3C). All the P(LA-co-BiBOEA) polymers were subjected to this postpolymerization modification (Figures 3D, S2) and the resulting P(LABiB-co-BiBOEA) macroinitiators were used in ATRP grafting-from to synthesize degradable BB polymers.
Figure 3.
(A) Reaction scheme for the postpolymerization modification of P(LA-co-BiBOEA) prepolymers with HOBiB to obtain P(LABiB-co-BiBOEA) macroinitiators. (B) 1H NMR spectra of P(LA-co-BiBOEA) (top) and P(LABiB-co-BiBOEA) (bottom) recorded in acetone-d6. (C) Overlaid SEC traces of P(LA-co-BiBOEA)140 and P(LABiB-co-BiBOEA)140, showing an increase in molecular weight following postpolymerization modification. (D) SEC traces corresponding to P(LABiB-co-BiBOEA) macroinitiators of varying DP (140, 220, 430).
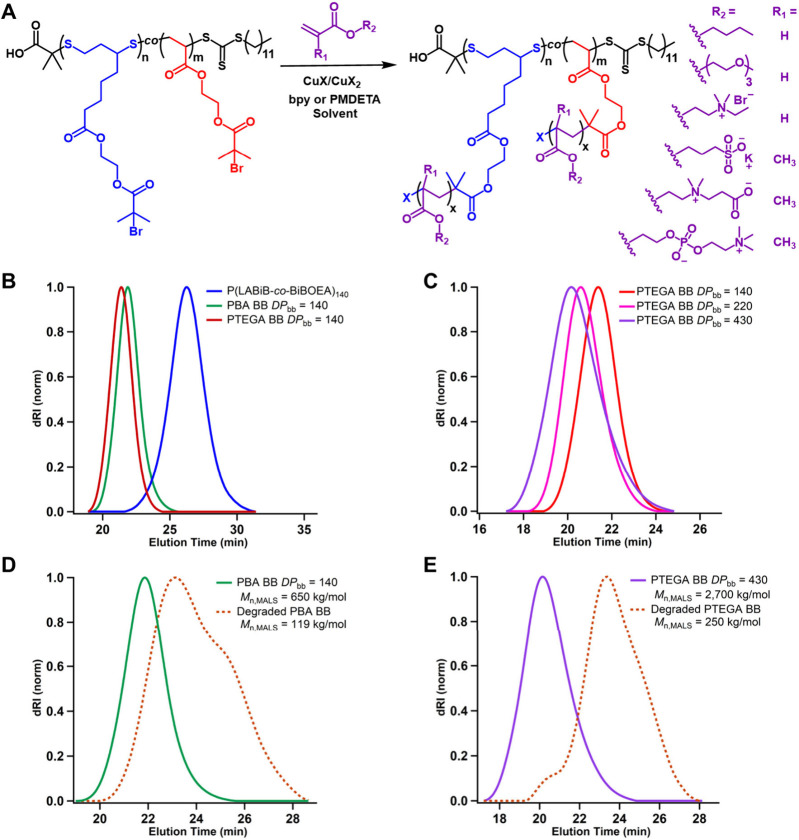
P(LABiB-co-BiBOEA)100–430 were used for ATRP grafting-from to obtain brushes with varying backbone chain lengths (DPbb). BA and tri(ethylene glycol) methyl ether acrylate (TEGA) were chosen as monomers (Figure 4A), with the latter serving to install hydrophilic side chains to provide water solubility. The grafting-from conditions were adapted from previously established normal ATRP conditions,37,38 with CuBr/CuBr2 catalyst and N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDTA) ligand in anisole at 60 °C. Using BA as the monomer, at molar ratios [BA]/[PMDTA]/[CuBr]/[CuBr2] = 400/0.6/0.53/0.03, the ATRP polymerizations reached moderate conversions of 12–27% following 23–24 h reaction times (Table 2), after which the polymerizations were stopped to maintain controlled dispersity and limit side chain length (DPsc). The resulting PBA brushes with DPbb = 140, 220, and 430 had respective molar masses of 650, 1390, and 2290 kg/mol, as measured by SEC-MALS, and controlled dispersities comparable to the corresponding macroinitiators (Đ = 1.30, 1.42, 1.49) (Figure S3). The ATRP grafting-from was carried out using TEGA as the monomer with [TEGA]/[PMDTA]/[CuBr]/[CuBr2] = 400/0.6/0.53/0.03, in anisole and at 60 °C, obtaining PTEGA BB. These polymerizations proceeded much more rapidly than with BA, achieving conversions of 10–12% after 3–4 h (Table 2). SEC-MALS analysis of PTEGA BB with varying backbone lengths of DPbb = 140, 220, and 430 revealed molar masses of 921, 1940, and 2700 kg/mol and Đ = 1.36, 1.37, 1.53, respectively (Figure 4C). The PTEGA BBs were water-soluble at concentrations up to 0.2% w/w, implying good biocompatibility that will be further enhanced by the degradability of the BB.
Figure 4.
(A) Reaction scheme of ATRP grafting-from BB synthesis using various (meth-)acrylate monomers. (B) SEC trace of P(LABiB-co-BiBOEA)140 macroinitiator overlaid with corresponding traces of PBA and PTEGA BB after ATRP grafting-from. (C) PTEGA BB polymers of varying backbone lengths (DPbb). (D) SEC traces showing degradation of PBA BB using 0.5 M dithiothreitol (DTT) in DMF at 22 °C, after 24 h. (E) SEC traces showing degradation of PTEGA BB using 0.1 M tris(2-carboxyethyl) phosphine (TCEP) in water at 22 °C, after 24 h.
Table 2. Characterization of Degradable BB Polymers Obtained using ATRP Grafting-From P(LABiB-co-BiBOEA).
| entry | MI DP | time (h) | monomer | conv.a (%) | Mn,MALSb (kg/mol) | Đb |
|---|---|---|---|---|---|---|
| 1 | 140 | 23 | BA | 12 | 650 | 1.30 |
| 2 | 220 | 22 | BA | 27 | 1390 | 1.42 |
| 3 | 430 | 24 | BA | 17 | 2290 | 1.49 |
| 4 | 140 | 3.0 | TEGA | 10 | 921 | 1.36 |
| 5 | 220 | 4.5 | TEGA | 12 | 1940 | 1.37 |
| 6 | 430 | 3.0 | TEGA | 12 | 2700 | 1.53 |
| 7 | 220 | 4.0 | DMEAA | 8.0 | 4780 | 1.28 |
| 8 | 220 | 3.0 | MPC | 38 | 4070 | 1.35 |
| 9 | 320 | 1.0 | CBMA | 50 | 3700 | 1.16 |
| 10 | 100 | 1.0 | SPMA | 24 | 537 | 1.31 |
Monomer conversion determined by 1H NMR spectroscopy.
Absolute number-average molar mass (Mn,MALS) and dispersity (Đ) were determined by SEC equipped with a multiangle light-scattering detector assuming 100% mass recovery, using DMF or aqueous buffer as eluent.
To expand the monomer scope of grafting-from ATRP and synthesize degradable brushes with charged and zwitterionic side chains bearing unique chemical and physical properties, several acrylate and methacrylate monomers were selected for grafting-from P(LABiB-co-BiBOEA) macroinitiators (Table 2 and Figure 4A). The quaternary ammonium-containing cationic N,N-dimethyl-N-ethylethylammonium acrylate (DMEAA) was used in ATRP grafting-from P(LABiB-co-BiBOEA)220, at ratios of [DMEAA]/[PMDTA]/[CuBr]/[CuBr2] = 400/0.6/0.5/0.03 and in DMSO as solvent. Within 2 h, the polymerization reached 8% conversion (Table 2), and the resulting BB polymer had a high molar mass (Mn,MALS = 4,780 kg/mol) with low dispersity (Đ = 1.28) (Figure S4). Furthermore, zwitterionic methacrylate monomers 2-methacryloyloxyethyl phosphorylcholine (MPC) and 3-[[2-(methacryloyloxy)ethyl]dimethylammonio] propionate (CBMA) were used in grafting-from, employing a previously reported CuCl/CuCl2 catalyst system with 2,2′-bipyridine (bpy) as ligand to ensure a controlled polymerization and prevent cross-link formation between BB structures.39,40 Zwitterionic monomers are of particular interest for biomedical applications due to their excellent antifouling properties, which minimize nonspecific protein adsorption and biofilm formation, making them ideal for applications such as drug delivery, biosensors, and implantable devices.41,42 Additionally, MPC-containing BB polymers have been reported to exhibit exceptional lubrication properties, mimicking the function of natural lubricants in articular cartilage, which reduces friction and wear under physiological conditions.39,43 However, these zwitterionic BB polymers, including MPC-based systems, have not yet been made degradable, limiting their potential for applications requiring controlled breakdown. When carried out at 50 °C in methanol/DMSO solvent mixture at [methacrylate]/[bpy]/[CuCl]/[CuCl2] ratios = 125/3.40/1.50/0.20, the ATRP with MPC reached 38% conversion. The molar mass of the PMPC BB was measured at 4070 kg/mol, and dispersity remained controlled at Đ = 1.35 (Figure S4). Similarly, the ATRP with CBMA reached 50% conversion in just 1 h at [methacrylate]/[bpy]/[CuCl]/[CuCl2] ratios = 150/3.40/1.50/0.20 using methanol/DMSO/acetonitrile solvent mixture at 40 °C, with the molar mass of the PCBMA BB being measured at 3,700 kg/mol, and its dispersity at Đ = 1.16. Finally, the anionic monomer 3-sulfopropyl methacrylate (SPMA) was used in ATRP grafting-from P(LABiB-co-BiBOEA)100 at ratios of [methacrylate]/[bpy]/[CuCl]/[CuCl2] = 150/3.40/1.50/0.20 in DMSO solvent and reached 24% conversion in 1h. The molar mass of the PSMPA BB was measured at 537 kg/mol, and its dispersity at Đ = 1.31.
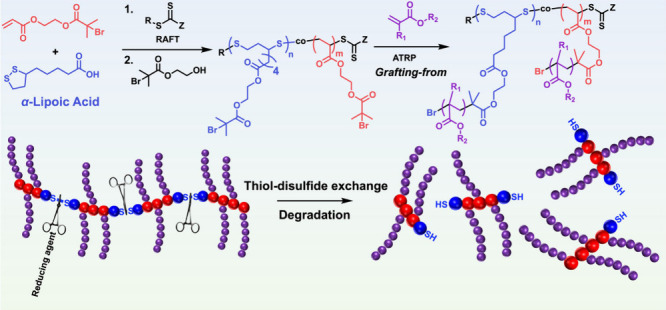
The degradation of the BBs was investigated using mild thiol reducing agents dithiothreitol (DTT) and TCEP. Using 0.5 M DTT in DMF at 22 °C, the PBA brush with DPbb = 140 (Mn,MALS = 650 kg/mol, Đ = 1.30) degraded to 18% of its original molar mass (Mn,MALS = 119 kg/mol, Đ = 1.53) after 24 h (Figure 4D). Aqueous TCEP (0.1 M) readily degraded PTEGA brush with DPbb = 430 (Mn,MALS = 2700 kg/mol, Đ = 1.53) at 22 °C to just 9% of its molar mass (Mn,MALS = 250 kg/mol, Đ = 1.87) after 24 h (Figure 4E). The ability of both reducing agents to degrade the BB confirmed the presence of disulfide bonds in the backbones, with the more complete degradation by TCEP reflecting its greater power as a reducing agent (Eo = −1.62 V) compared to DTT (Eo = −0.33 V) and the lack of disulfide bond reformation after reduction.44,45 These favorable degradation profiles using mild reducing agents further support the future application of the BB polymers in applications requiring biocompatibility and degradation after use.
In summary, we have developed a synthetic strategy for degradable BB polymers with backbone disulfide bonds. α-Lipoic acid (LA) was copolymerized through RAFT with ATRP inimer 2-(2-(bromoisobutyryl)oxy)ethyl acrylate (BiBOEA) to yield prepolymers that could be further modified with 2-(hydroxyethyl) 2-bromoisobutyrate (HOBiB) to obtain ATRP macroinitiators with high initiator side chain density. ATRP grafting-from was employed to synthesize BB polymers with varying molecular weight using n-butyl acrylate (BA), as well as hydrophilic tri(ethylene glycol) methyl ether acrylate (TEGA) for water solubility. In addition, a cationic acrylate and zwitterionic methacrylates were used as monomers in ATRP grafting-from, entailing a broad monomer scope for obtaining water-soluble degradable BBs with charged side chains for biomedical applications. Finally, the BB polymers were degraded under mild thiol-reducing conditions using dithiothreitol (DTT) and 2-tris(2-carboxyethyl) phosphine, confirming the presence of degradable disulfide units in the backbone that enhance the biocompatibility of the BBs.
Acknowledgments
The financial support from NSF (DMR 2202747) is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmacrolett.4c00839.
Chemicals, instrumentation, synthetic procedures, size-exclusion chromatograms, and 1H NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rathgeber S.; Pakula T.; Wilk A.; Matyjaszewski K.; Beers K. L. On the shape of bottle-brush macromolecules: Systematic variation of architectural parameters. J. Chem. Phys. 2005, 122 (12), na. 10.1063/1.1860531. [DOI] [PubMed] [Google Scholar]
- Li Z.; Tang M.; Liang S.; Zhang M.; Biesold G. M.; He Y.; Hao S.-M.; Choi W.; Liu Y.; Peng J.; Lin Z. Bottlebrush polymers: From controlled synthesis, self-assembly, properties to applications. Prog. Polym. Sci. 2021, 116, 101387. 10.1016/j.progpolymsci.2021.101387. [DOI] [Google Scholar]
- Verduzco R.; Li X.; Pesek S. L.; Stein G. E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 2015, 44 (8), 2405–2420. 10.1039/C4CS00329B. [DOI] [PubMed] [Google Scholar]
- Xie G.; Martinez M. R.; Olszewski M.; Sheiko S. S.; Matyjaszewski K. Molecular bottlebrushes as novel materials. Biomacromolecules 2019, 20 (1), 27–54. 10.1021/acs.biomac.8b01171. [DOI] [PubMed] [Google Scholar]
- Li X.; Prukop S. L.; Biswal S. L.; Verduzco R. Surface properties of bottlebrush polymer thin films. Macromolecules 2012, 45 (17), 7118–7127. 10.1021/ma301046n. [DOI] [Google Scholar]
- Rekha Rout S.; Kenguva G.; Mansuri S.; Manu K. R.; Dandela R.; Pramanik N. B. Bottlebrush polymers via ring-opening metathesis polymerization (ROMP): Synthesis, properties and applications. Eur. Polym. J. 2024, 221, 113546 10.1016/j.eurpolymj.2024.113546. [DOI] [Google Scholar]
- Vatankhah-Varnosfaderani M.; Daniel W. F. M.; Everhart M. H.; Pandya A. A.; Liang H.; Matyjaszewski K.; Dobrynin A. V.; Sheiko S. S. Mimicking biological stress–strain behaviour with synthetic elastomers. Nature 2017, 549 (7673), 497–501. 10.1038/nature23673. [DOI] [PubMed] [Google Scholar]
- Daniel W. F. M.; Burdyńska J.; Vatankhah-Varnoosfaderani M.; Matyjaszewski K.; Paturej J.; Rubinstein M.; Dobrynin A. V.; Sheiko S. S. Solvent-free, supersoft and superelastic bottlebrush melts and networks. Nat. Mater. 2016, 15 (2), 183–189. 10.1038/nmat4508. [DOI] [PubMed] [Google Scholar]
- Lee H.-i.; Pietrasik J.; Sheiko S. S.; Matyjaszewski K. Stimuli-responsive molecular brushes. Prog. Polym. Sci. 2010, 35 (1), 24–44. 10.1016/j.progpolymsci.2009.11.002. [DOI] [Google Scholar]
- Sheiko S. S.; Sumerlin B. S.; Matyjaszewski K. Cylindrical molecular brushes: Synthesis, characterization, and properties. Prog. Polym. Sci. 2008, 33 (7), 759–785. 10.1016/j.progpolymsci.2008.05.001. [DOI] [Google Scholar]
- Baker S. L.; Kaupbayeva B.; Lathwal S.; Das S. R.; Russell A. J.; Matyjaszewski K. Atom transfer radical polymerization for biorelated hybrid materials. Biomacromolecules 2019, 20 (12), 4272–4298. 10.1021/acs.biomac.9b01271. [DOI] [PubMed] [Google Scholar]
- Detappe A.; Nguyen H. V. T.; Jiang Y.; Agius M. P.; Wang W.; Mathieu C.; Su N. K.; Kristufek S. L.; Lundberg D. J.; Bhagchandani S.; Ghobrial I. M.; Ghoroghchian P. P.; Johnson J. A. Molecular bottlebrush prodrugs as mono- and triplex combination therapies for multiple myeloma. Nat. Nanotechnol. 2023, 18 (2), 184–192. 10.1038/s41565-022-01310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F.; Chen P.; Wang D.; Sun Y.; Ren M.; Wang Y.; Cao X.; Zhang L.; Fang Y.; Tan X.; Lu H.; Cai J.; Lu X.; Zhang K. Bottlebrush polymer-conjugated melittin exhibits enhanced antitumor activity and better safety profile. ACS Appl. Mater. Interfaces 2021, 13 (36), 42533–42542. 10.1021/acsami.1c14285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müllner M. Molecular Polymer Brushes in Nanomedicine. Macromol. Chem. Phys. 2016, 217 (20), 2209–2222. 10.1002/macp.201600086. [DOI] [Google Scholar]
- Müllner M.; Yang K.; Kaur A.; New E. J. Aspect-ratio-dependent interaction of molecular polymer brushes and multicellular tumour spheroids. Polym. Chem. 2018, 9 (25), 3461–3465. 10.1039/C8PY00703A. [DOI] [Google Scholar]
- Rabanel J.-M.; Mirbagheri M.; Olszewski M.; Xie G.; Le Goas M.; Latreille P.-L.; Counil H.; Hervé V.; Silva R. O.; Zaouter C.; Adibnia V.; Acevedo M.; Servant M. J.; Martinez V. A.; Patten S. A.; Matyjaszewski K.; Ramassamy C.; Banquy X. Deep Tissue Penetration of Bottle-Brush Polymers via Cell Capture Evasion and Fast Diffusion. ACS Nano 2022, 16 (12), 21583–21599. 10.1021/acsnano.2c10554. [DOI] [PubMed] [Google Scholar]
- Shieh P.; Nguyen H. V. T.; Johnson J. A. Tailored silyl ether monomers enable backbone-degradable polynorbornene-based linear, bottlebrush and star copolymers through ROMP. Nat. Chem. 2019, 11 (12), 1124–1132. 10.1038/s41557-019-0352-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Wen Y.; Wang Y.; Li W.; Lu X.; You W. 7-Oxa-2,3-Diazanorbornene: A One-Step Accessible Monomer for Living Ring-Opening Metathesis Polymerization to Produce Backbone-Biodegradable Polymers. CCS Chemistry 2024, 6, 2305–2317. 10.31635/ccschem.024.202303697. [DOI] [Google Scholar]
- un Nisa Q.; Theobald W.; Hepburn K. S.; Riddlestone I.; Bingham N. M.; Kopeć M.; Roth P. J. Degradable linear and bottlebrush thioester-functional copolymers through atom-transfer radical ring-opening copolymerization of a thionolactone. Macromolecules 2022, 55 (17), 7392–7400. 10.1021/acs.macromol.2c01317. [DOI] [Google Scholar]
- Lee D.; Wang H.; Jiang S.-Y.; Verduzco R. Versatile light-mediated synthesis of degradable bottlebrush polymers using α-lipoic acid. Angew. Chem., Int. Ed. Engl. 2024, 63 (48), e202409323. 10.1002/anie.202409323. [DOI] [PubMed] [Google Scholar]
- Morris P. T.; Watanabe K.; Albanese K. R.; Kent G. T.; Gupta R.; Gerst M.; Read de Alaniz J.; Hawker C. J.; Bates C. M. Scalable Synthesis of Degradable Copolymers Containing α-Lipoic Acid via Miniemulsion Polymerization. J. Am. Chem. Soc. 2024, 146 (44), 30662–30667. 10.1021/jacs.4c12438. [DOI] [PubMed] [Google Scholar]
- Albanese K. R.; Morris P. T.; Read de Alaniz J.; Bates C. M.; Hawker C. J. Controlled-radical polymerization of α-lipoic acid: A general route to degradable vinyl copolymers. J. Am. Chem. Soc. 2023, 145 (41), 22728–22734. 10.1021/jacs.3c08248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu S.; Rempson C. M.; Puche V.; Zhao B.; Zhang F. Construction of disulfide containing redox-responsive polymeric nanomedicine. Methods 2022, 199, 67–79. 10.1016/j.ymeth.2021.12.011. [DOI] [PubMed] [Google Scholar]
- Zhang R.; Nie T.; Fang Y.; Huang H.; Wu J. Poly(disulfide)s: From synthesis to drug delivery. Biomacromolecules 2022, 23 (1), 1–19. 10.1021/acs.biomac.1c01210. [DOI] [PubMed] [Google Scholar]
- Cho H. Y.; Srinivasan A.; Hong J.; Hsu E.; Liu S.; Shrivats A.; Kwak D.; Bohaty A. K.; Paik H.-j.; Hollinger J. O.; Matyjaszewski K. Synthesis of Biocompatible PEG-Based Star Polymers with Cationic and Degradable Core for siRNA Delivery. Biomacromolecules 2011, 12 (10), 3478–3486. 10.1021/bm2006455. [DOI] [PubMed] [Google Scholar]
- Fu S.; Zheng A.; Wang L.; Chen J.; Zhao B.; Zhang X.; McKenzie V. A. A.; Yang Z.; Leblanc R. M.; Prabhakar R.; Zhang F. Tuneable redox-responsive albumin-hitchhiking drug delivery to tumours for cancer treatment. J. Mater. Chem. B Mater. Biol. Med. 2024, 12 (27), 6563–6569. 10.1039/D4TB00751D. [DOI] [PubMed] [Google Scholar]
- Jazani A. M.; Arezi N.; Shetty C.; Hong S. H.; Li H.; Wang X.; Oh J. K. Tumor-targeting intracellular drug delivery based on dual acid/reduction-degradable nanoassemblies with ketal interface and disulfide core locations. Polym. Chem. 2019, 10 (22), 2840–2853. 10.1039/C9PY00352E. [DOI] [Google Scholar]
- Li Y.; Nese A.; Lebedeva N. V.; Davis T.; Matyjaszewski K.; Sheiko S. S. Molecular Tensile Machines: Intrinsic Acceleration of Disulfide Reduction by Dithiothreitol. J. Am. Chem. Soc. 2011, 133 (43), 17479–17484. 10.1021/ja207491r. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Jia Y.; Wu Q.; Moore J. S. Architecture-controlled ring-opening polymerization for dynamic covalent poly(disulfide)s. J. Am. Chem. Soc. 2019, 141 (43), 17075–17080. 10.1021/jacs.9b08957. [DOI] [PubMed] [Google Scholar]
- Zhang X.; Waymouth R. M. 1,2-dithiolane-derived dynamic, covalent materials: Cooperative self-assembly and reversible cross-linking. J. Am. Chem. Soc. 2017, 139 (10), 3822–3833. 10.1021/jacs.7b00039. [DOI] [PubMed] [Google Scholar]
- Levkovskyi I. O.; Mochizuki S.; Zheng A.; Zhang X.; Zhang F. Lipoic acid-based poly(disulfide)s: Synthesis and biomedical applications. Nano TransMed. 2023, 2 (2–3), 100006 10.1016/j.ntm.2023.100006. [DOI] [Google Scholar]
- Albanese K. R.; Read de Alaniz J.; Hawker C. J.; Bates C. M. From health supplement to versatile monomer: Radical ring-opening polymerization and depolymerization of α-lipoic acid. Polymer 2024, 304, 127167 10.1016/j.polymer.2024.127167. [DOI] [Google Scholar]
- Tang H.; Tsarevsky N. V. Lipoates as building blocks of sulfur-containing branched macromolecules. Polym. Chem. 2015, 6 (39), 6936–6945. 10.1039/C5PY01005E. [DOI] [Google Scholar]
- Matyjaszewski K.; Gaynor S. G.; Müller A. H. E. Preparation of Hyperbranched Polyacrylates by Atom Transfer Radical Polymerization. 2. Kinetics and Mechanism of Chain Growth for the Self-Condensing Vinyl Polymerization of 2-((2-Bromopropionyl)oxy)ethyl Acrylate. Macromolecules 1997, 30 (23), 7034–7041. 10.1021/ma970634z. [DOI] [Google Scholar]
- Lorandi F.; Fantin M.; Matyjaszewski K. Atom Transfer Radical Polymerization: A Mechanistic Perspective. J. Am. Chem. Soc. 2022, 144 (34), 15413–15430. 10.1021/jacs.2c05364. [DOI] [PubMed] [Google Scholar]
- Matyjaszewski K. Advanced Materials by Atom Transfer Radical Polymerization. Adv. Mater. 2018, 30 (23), 1706441. 10.1002/adma.201706441. [DOI] [PubMed] [Google Scholar]
- Beers K. L.; Gaynor S. G.; Matyjaszewski K.; Sheiko S. S.; Möller M. The Synthesis of Densely Grafted Copolymers by Atom Transfer Radical Polymerization. Macromolecules 1998, 31 (26), 9413–9415. 10.1021/ma981402i. [DOI] [Google Scholar]
- Grigoriadis C.; Nese A.; Matyjaszewski K.; Pakula T.; Butt H.-J.; Floudas G. Dynamic homogeneity by architectural design - bottlebrush polymers. Macromol. Chem. Phys. 2012, 213 (13), 1311–1320. 10.1002/macp.201200064. [DOI] [Google Scholar]
- Banquy X.; Burdyńska J.; Lee D. W.; Matyjaszewski K.; Israelachvili J. Bioinspired bottle-brush polymer exhibits low friction and Amontons-like behavior. J. Am. Chem. Soc. 2014, 136 (17), 6199–6202. 10.1021/ja501770y. [DOI] [PubMed] [Google Scholar]
- Saha B.; Boykin J.; Chung H. Unveiling the architectural impact on the salt-tunable adhesion performance and toughness of polyzwitterions. J. Am. Chem. Soc. 2024, 146 (33), 23467–23475. 10.1021/jacs.4c06877. [DOI] [PubMed] [Google Scholar]
- Lee J.; Tang Y.; Cureño Hernandez K. E.; Kim S.; Lee R.; Cartwright Z.; Pochan D. J.; Herrera-Alonso M. Ultrastable and redispersible zwitterionic bottlebrush micelles for drug delivery. ACS Appl. Mater. Interfaces 2024, na. 10.1021/acsami.4c10968. [DOI] [PubMed] [Google Scholar]
- Milatz R.; Duvigneau J.; Vancso G. J. Dopamine-based copolymer bottlebrushes for functional adhesives: Synthesis, characterization, and applications in surface engineering of antifouling polyethylene. ACS Appl. Mater. Interfaces 2023, 15 (28), 34023–34030. 10.1021/acsami.3c05124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham D. A.; Wang C.-S.; Séguy L.; Zhang H.; Benbabaali S.; Faivre J.; Sim S.; Xie G.; Olszewski M.; Rabanel J.-M.; Moldovan F.; Matyjaszewski K.; Banquy X. Bioinspired Bottlebrush Polymers Effectively Alleviate Frictional Damage Both In Vitro and In Vivo. Adv. Mater. 2024, 36 (25), 2401689. 10.1002/adma.202401689. [DOI] [PubMed] [Google Scholar]
- Bartoccini F.; Retini M.; Crinelli R.; Menotta M.; Fraternale A.; Piersanti G. Dithiol based on l-cysteine and cysteamine as a disulfide-reducing agent. J. Org. Chem. 2022, 87 (15), 10073–10079. 10.1021/acs.joc.2c01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajes K.; Walker K. A.; Hadam S.; Zabihi F.; Rancan F.; Vogt A.; Haag R. Redox-responsive nanocarrier for controlled release of drugs in inflammatory skin diseases. Pharmaceutics 2021, 13 (1), 37. 10.3390/pharmaceutics13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.