Abstract
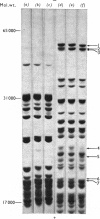
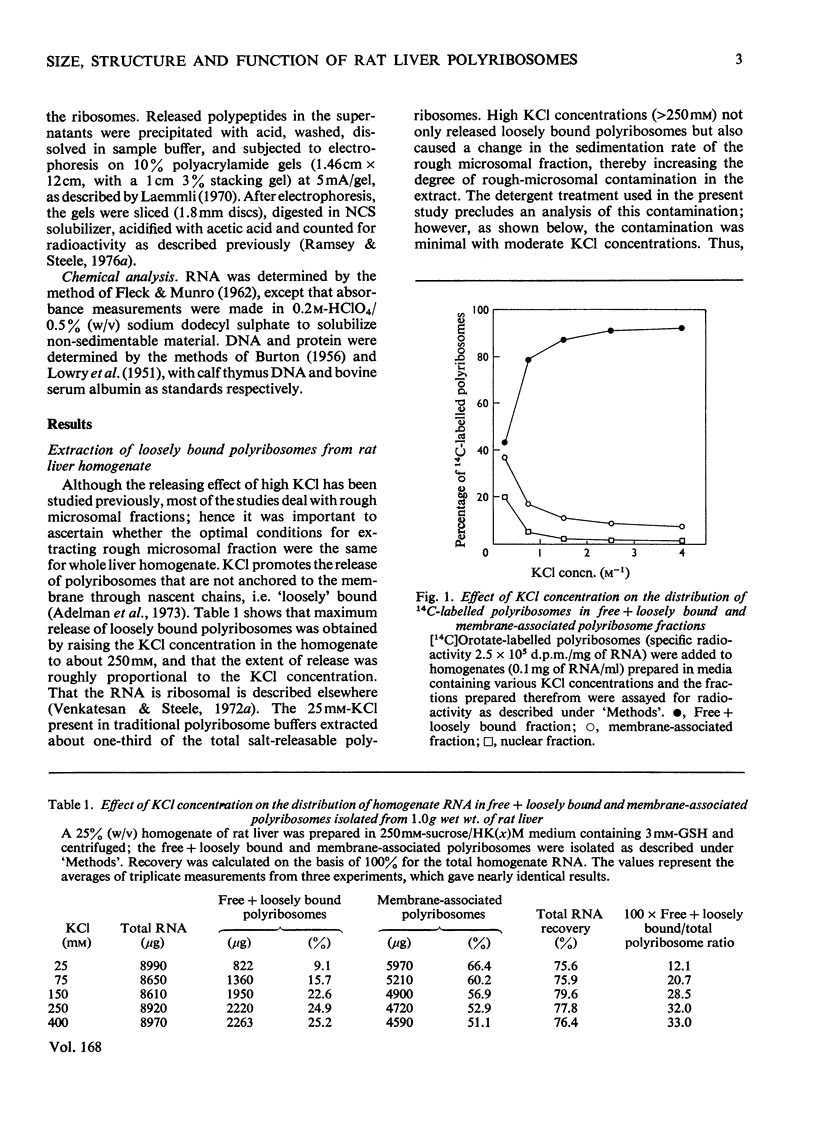
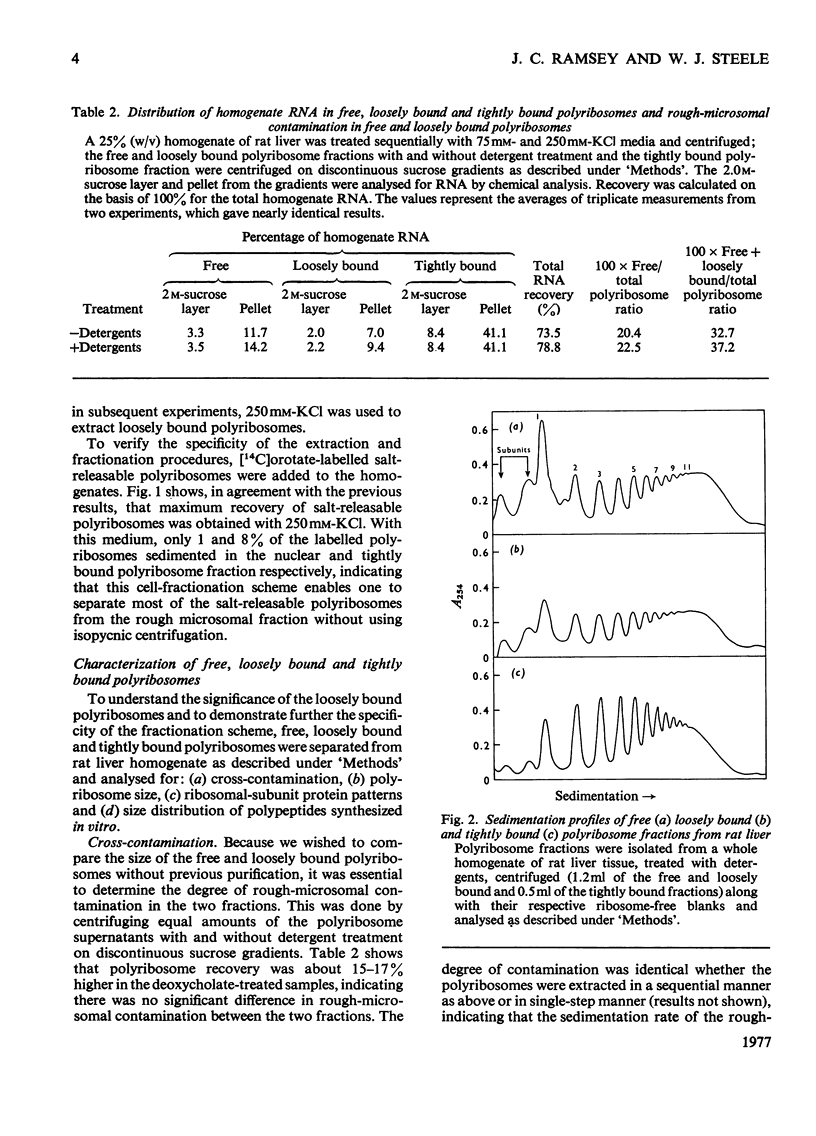
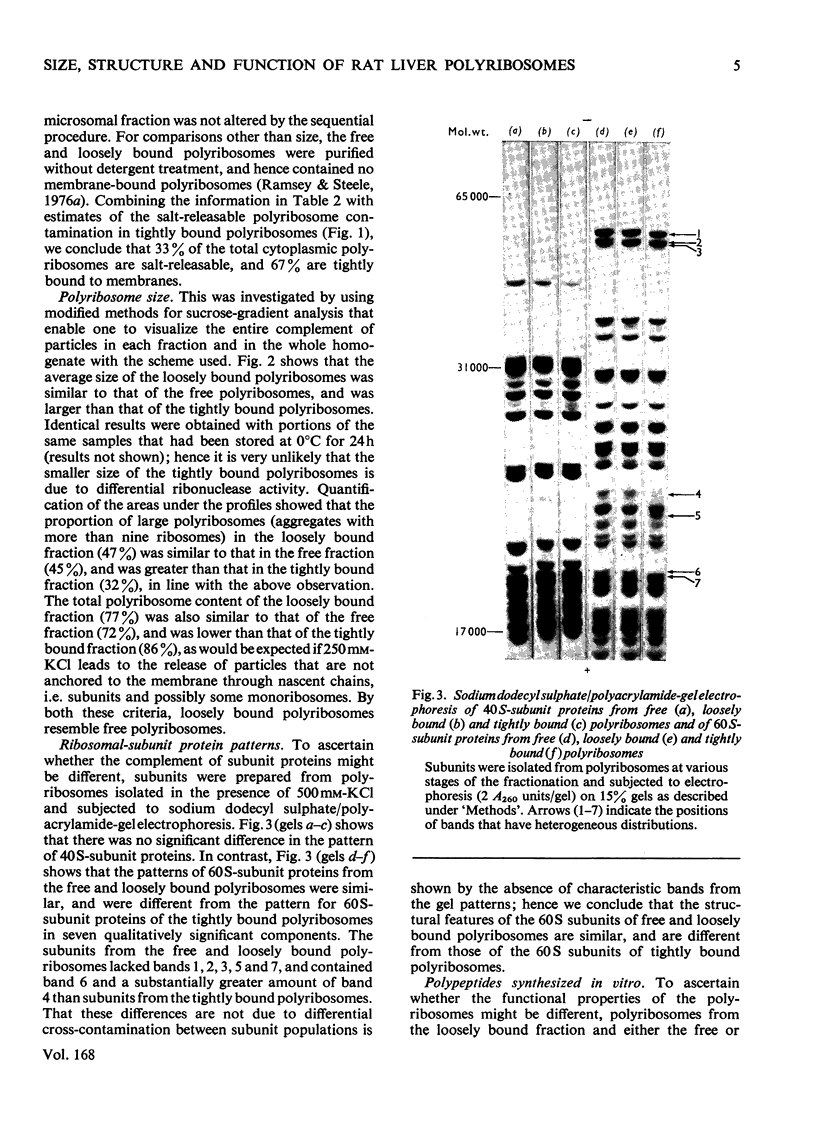
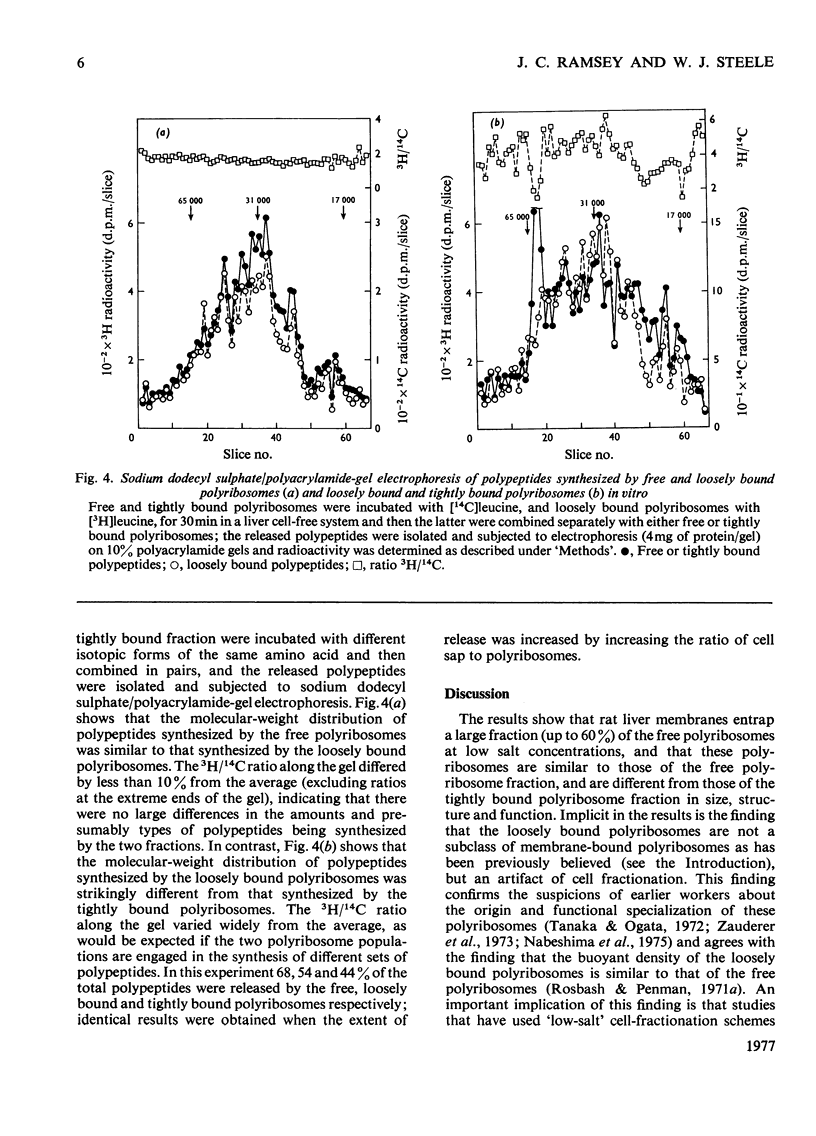
Free loosely bound and tightly bound polyribosomes were separated from rat liver homogenate by salt extraction followed by differential centrifugation, and several of their structural and functional properties were compared to resolve the existence of loosely bound polyribosomes and verify the specificity of the separation. The free and loosely bound polyribosomes have similar sedimentation profiles and polyribosome contents, their subunit proteins have similar electrophoretic patterns and their products of protein synthesis in vitro show a close correspondence in size and amounts synthesized. In contrast, the tightly bound polyribosomes have different properties from those of the free and loosely bound polyribosomes; their average size is significantly smaller; their polyribosome content is higher; their 60 S-subunit proteins lack two components and contain four or more components not found elsewhere; their products of protein synthesis in vitro differ in size and amounts synthesized. These observations show that rat liver membranes entrap a large fraction of the free polyribosomes at low salt concentrations and that these polyribosomes are similar to those of the free-polyribosome fraction and are different from those of the tightly bound polyribosome fraction in size, structure and function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelman M. R., Sabatini D. D., Blobel G. Ribosome-membrane interaction. Nondestructive disassembly of rat liver rough microsomes into ribosomal and membranous components. J Cell Biol. 1973 Jan;56(1):206–229. doi: 10.1083/jcb.56.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., BENEDETTI E. L. PREPARATION OF RAT-LIVER POLYSOMES WITHOUT THE UTILIZATION OF DETERGENT. Biochim Biophys Acta. 1964 May 18;87:177–180. doi: 10.1016/0926-6550(64)90064-7. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiberg I., Zauderer M., Baglioni C. Reversible disaggregation by NaF of membrane-bound polyribosomes of mouse myeloma cells in tissue culture. Biochim Biophys Acta. 1972 May 29;269(3):453–464. doi: 10.1016/0005-2787(72)90133-5. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Studies on free and membrane-bound ribosomes in rat liver. I. Distribution as related to total cellular RNA. J Mol Biol. 1967 Jun 14;26(2):279–292. doi: 10.1016/0022-2836(67)90297-5. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. Dissociation of mammalian polyribosomes into subunits by puromycin. Proc Natl Acad Sci U S A. 1971 Feb;68(2):390–394. doi: 10.1073/pnas.68.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese D., Blobel G., Sabatini D. D. In vitro exchange of ribosomal subunits between free and membrane-bound ribosomes. J Mol Biol. 1973 Mar 15;74(4):415–438. doi: 10.1016/0022-2836(73)90037-5. [DOI] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Hanna N., Bellemare G., Godin C. Free and membrane-bound ribosomes. I. Separation by two-dimensional gel electrophoresis of proteins from rat liver monosomes. Biochim Biophys Acta. 1973 Nov 26;331(1):141–145. [PubMed] [Google Scholar]
- Hoffman W. L., Ilan J. Analysis by two-dimensional polyacrylamide gel electrophoresis of liver ribosomal subunnit proteins obtained from free and membrane-bound polysomes of unfasted animals. Biochim Biophys Acta. 1977 Feb 3;474(3):411–424. doi: 10.1016/0005-2787(77)90270-2. [DOI] [PubMed] [Google Scholar]
- Kabat D. Phosphorylation of ribosomal proteins in rabbit reticulocytes. Characterization and regulatory aspects. Biochemistry. 1970 Oct 13;9(21):4160–4175. doi: 10.1021/bi00823a019. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Attachment of ribosomes to membranes during polysome formation in mouse sarcoma 180 cells. J Cell Biol. 1971 Jun;49(3):683–691. doi: 10.1083/jcb.49.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew C. C., Gornall A. G. Acetylation of ribosomal proteins. I. Characterization and properties of rat liver ribosomal proteins. J Biol Chem. 1973 Feb 10;248(3):977–983. [PubMed] [Google Scholar]
- McCarty K. S., Jr, Vollmer R. T., McCarty K. S. Improved computer program data for the resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1974 Sep;61(1):165–183. doi: 10.1016/0003-2697(74)90343-1. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y. I., Tsurugi K., Ogata K. Preferential biosynthesis of ribosomal structural proteins by free and loosely bound polysomes from regenerating rat liver. Biochim Biophys Acta. 1975 Nov 18;414(1):30–43. doi: 10.1016/0005-2787(75)90123-9. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. A procedure for the quantitative recovery of homogeneous populations of undegraded free and bound polysomes from rat liver. Biochemistry. 1976 Apr 20;15(8):1704–1712. doi: 10.1021/bi00653a018. [DOI] [PubMed] [Google Scholar]
- Ramsey J. C., Steele W. J. Effect of starvation of the distribution of free and membrane-bound ribosomes in rat liver and on the content of phospholipid and glycogen in purified ribosomes. Biochim Biophys Acta. 1976 Oct 18;447(3):312–318. doi: 10.1016/0005-2787(76)90054-x. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cells. I. The two classes of membrane-associated ribosomes. J Mol Biol. 1971 Jul 28;59(2):227–241. doi: 10.1016/0022-2836(71)90048-9. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Penman S. Membrane-associated protein synthesis of mammalian cells. II. Isopycnic separation of membrane-bound polyribosomes. J Mol Biol. 1971 Jul 28;59(2):243–253. doi: 10.1016/0022-2836(71)90049-0. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Ogata K. Two classes of membrane-bound ribosomes in rat liver cells and their albumin synthesizing activity. Biochem Biophys Res Commun. 1972 Nov 15;49(4):1069–1074. doi: 10.1016/0006-291x(72)90321-x. [DOI] [PubMed] [Google Scholar]
- Venkatesan N., Steele W. J. Free and membrane-bound polysomes of rat liver: separation in nearly quantitative yield and analysis of structure and function. Biochim Biophys Acta. 1972 Dec 22;287(3):526–537. doi: 10.1016/0005-2787(72)90298-5. [DOI] [PubMed] [Google Scholar]
- Venkatesan N., Steele W. J. Isolation of ribosomes from post-nuclear fraction of rat liver in nearly quantitative yield. Biochim Biophys Acta. 1972 Sep 14;277(3):646–650. doi: 10.1016/0005-2787(72)90109-8. [DOI] [PubMed] [Google Scholar]
- WEBB T. E., BLOBEL G., POTTER V. R. POLYRIBOSOMES IN RAT TISSUES. I. A STUDY OF IN VIVO PATTERNS IN LIVER AND HEPATOMAS. Cancer Res. 1964 Aug;24:1229–1237. [PubMed] [Google Scholar]
- Zauderer M., Liberti P., Baglioni C. Distribution of histone messenger RNA among free and membrane-associated polyribosomes of a mouse myeloma cell line. J Mol Biol. 1973 Sep 25;79(3):577–586. doi: 10.1016/0022-2836(73)90407-5. [DOI] [PubMed] [Google Scholar]