Abstract
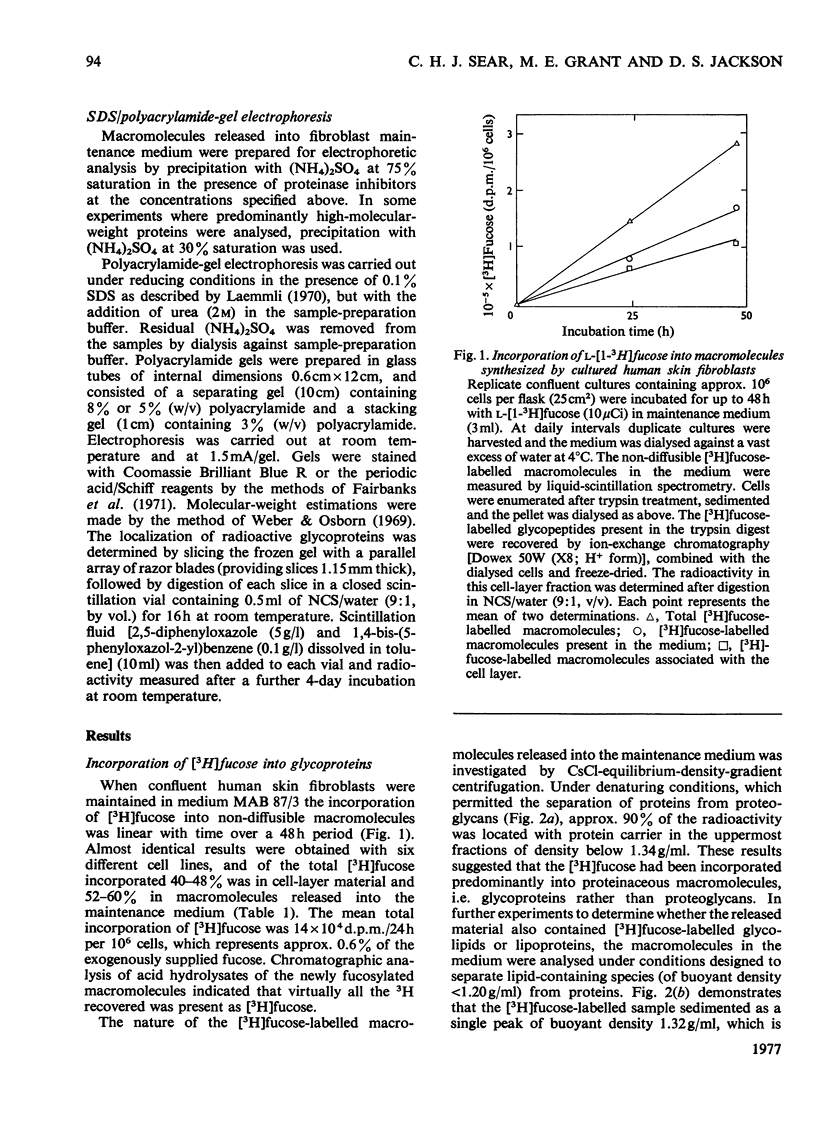
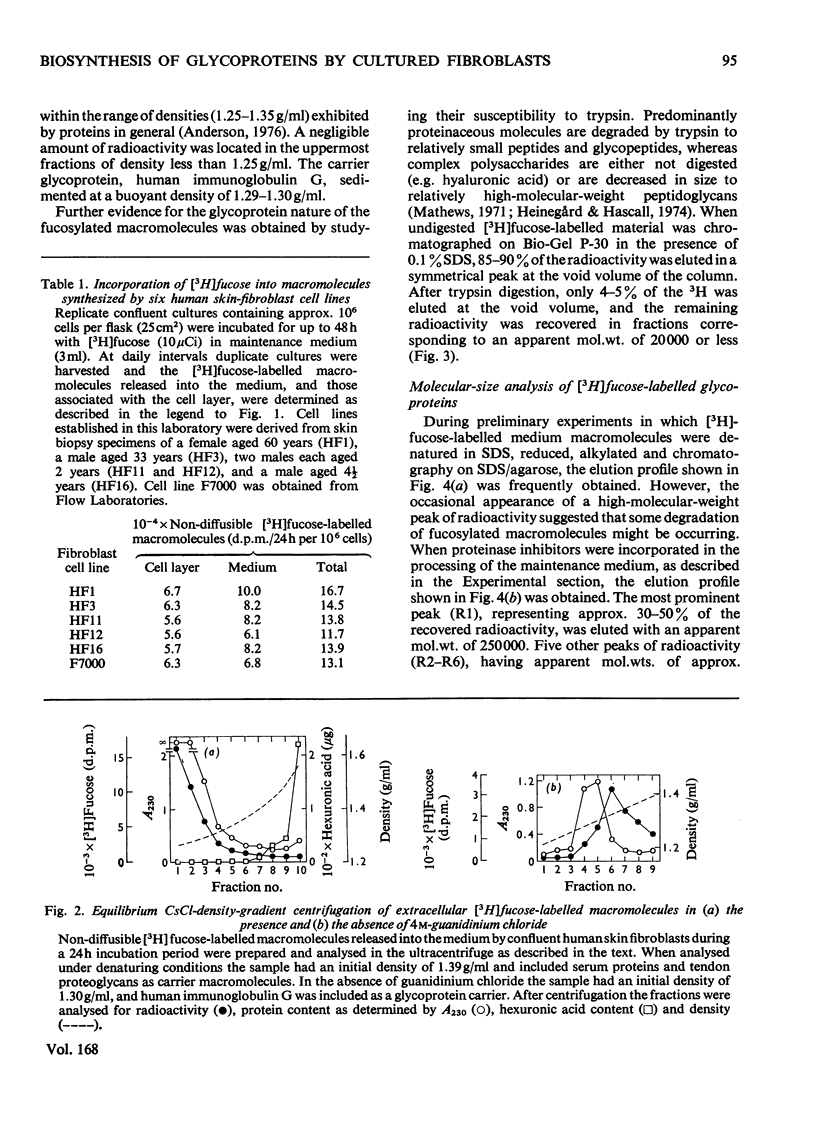
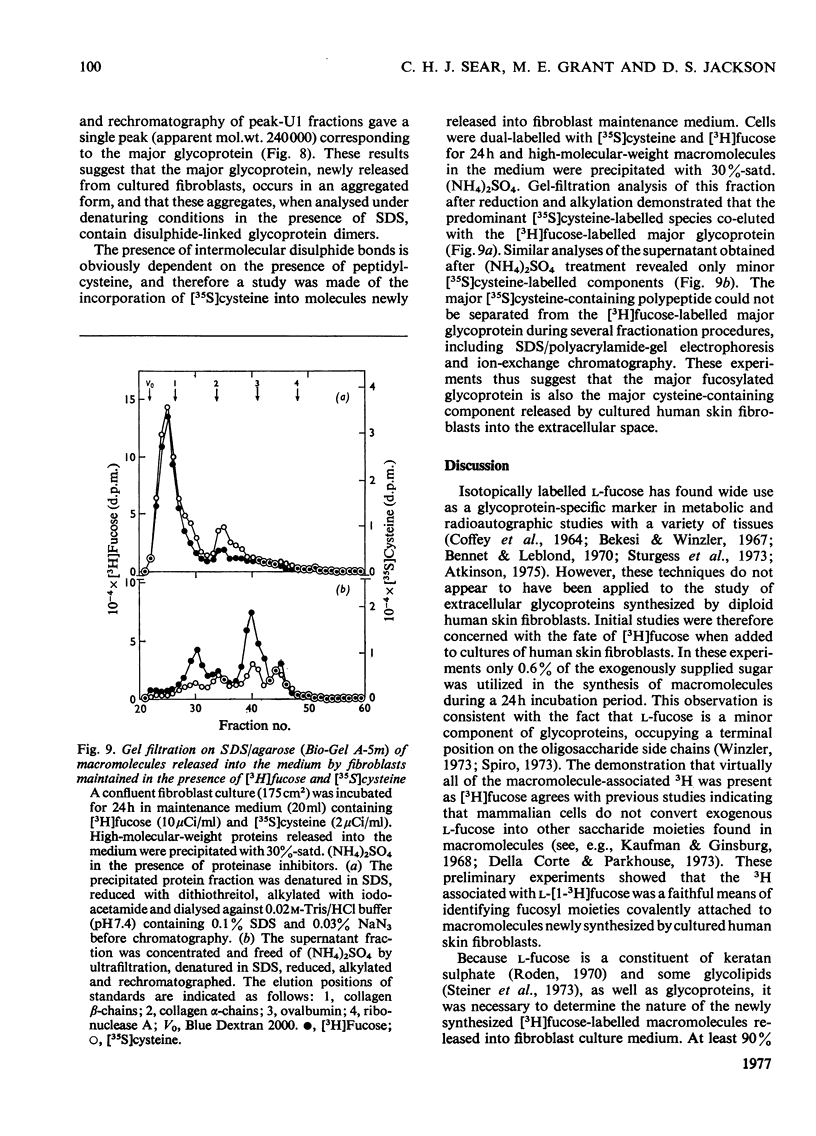
1. Confluent human skin fibroblasts maintained in a chemically defined medium incorporate l-[1-3H]fucose in a linear manner with time into non-diffusible macromolecules for up to 48h. Chromatographic analysis demonstrated that virtually all the macromolecule-associated 3H was present as [3H]fucose. 2. Equilibrium CsCl-density-gradient centrifugation established that [3H]fucose-labelled macromolecules released into the medium were predominantly glycoproteins. Confirmation of this finding was provided by molecular-size analyses of the [3H]fucose-labelled material before and after trypsin digestion. 3. The [3H]fucose-labelled glycoproteins released into fibroblast culture medium were analysed by gel-filtration chromatography and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis. These techniques demonstrated that the major fucosylated glycoprotein had an apparent mol.wt. of 230000–250000; several minor labelled species were also detected. 4. Dual-labelling experiments with [3H]fucose and 14C-labelled amino acids indicated that the major fucosylated glycoprotein was synthesized de novo by cultured fibroblasts. The non-collagenous nature of this glycoprotein was established by three independent methods. 5. Gel-filtration analysis before and after reduction with dithiothreitol showed that the major glycoprotein occurs as a disulphide-bonded dimer when analysed under denaturing conditions. Further experiments demonstrated that this glycoprotein was the predominant labelled species released into the medium when fibroblasts were incubated with [35S]cysteine. 6. The relationship between the major fucosylated glycoprotein and a glycoprotein, or group of glycoproteins, variously known as fibronectin, LETS protein, cell-surface protein etc., is discussed.
Full text
PDF


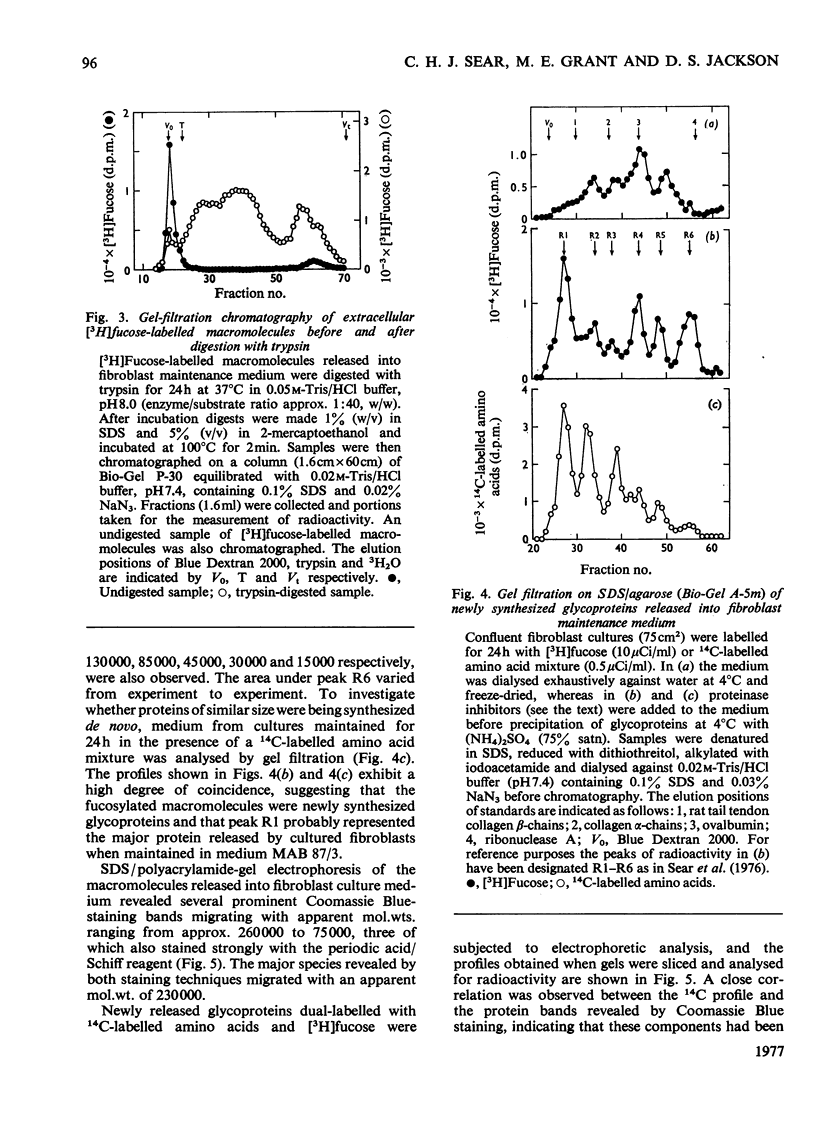
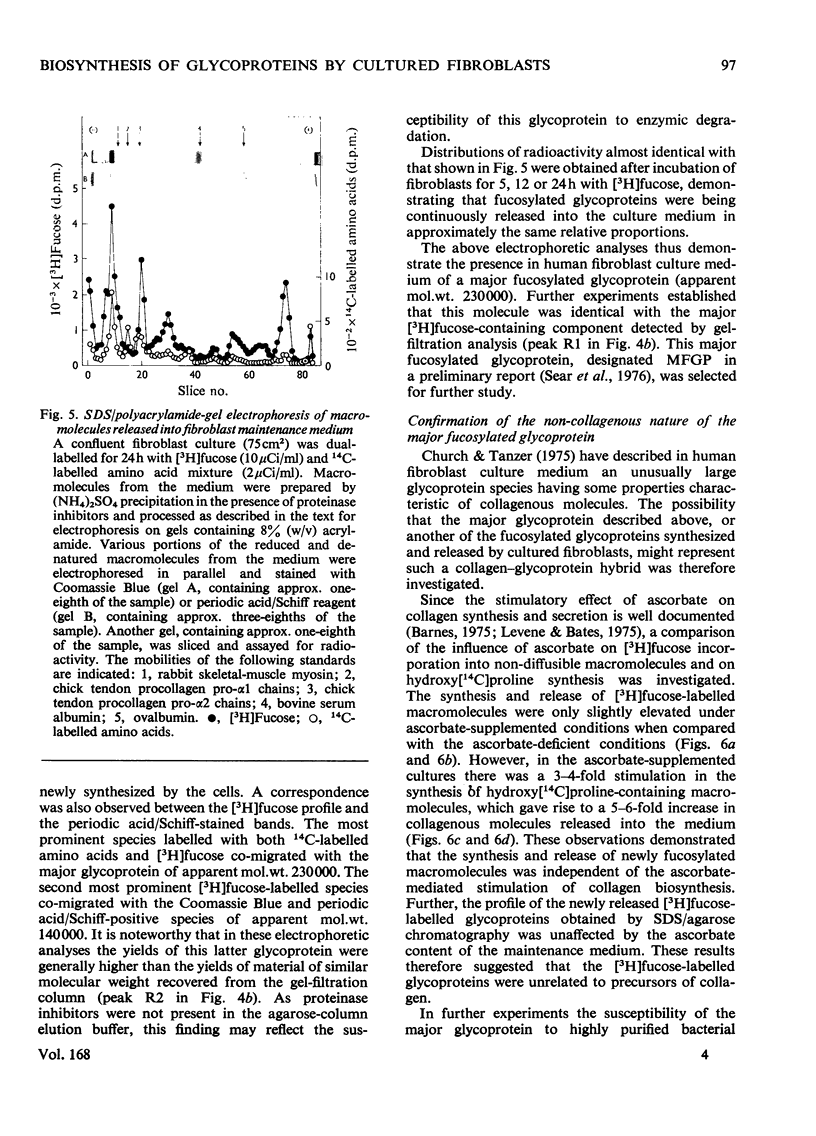
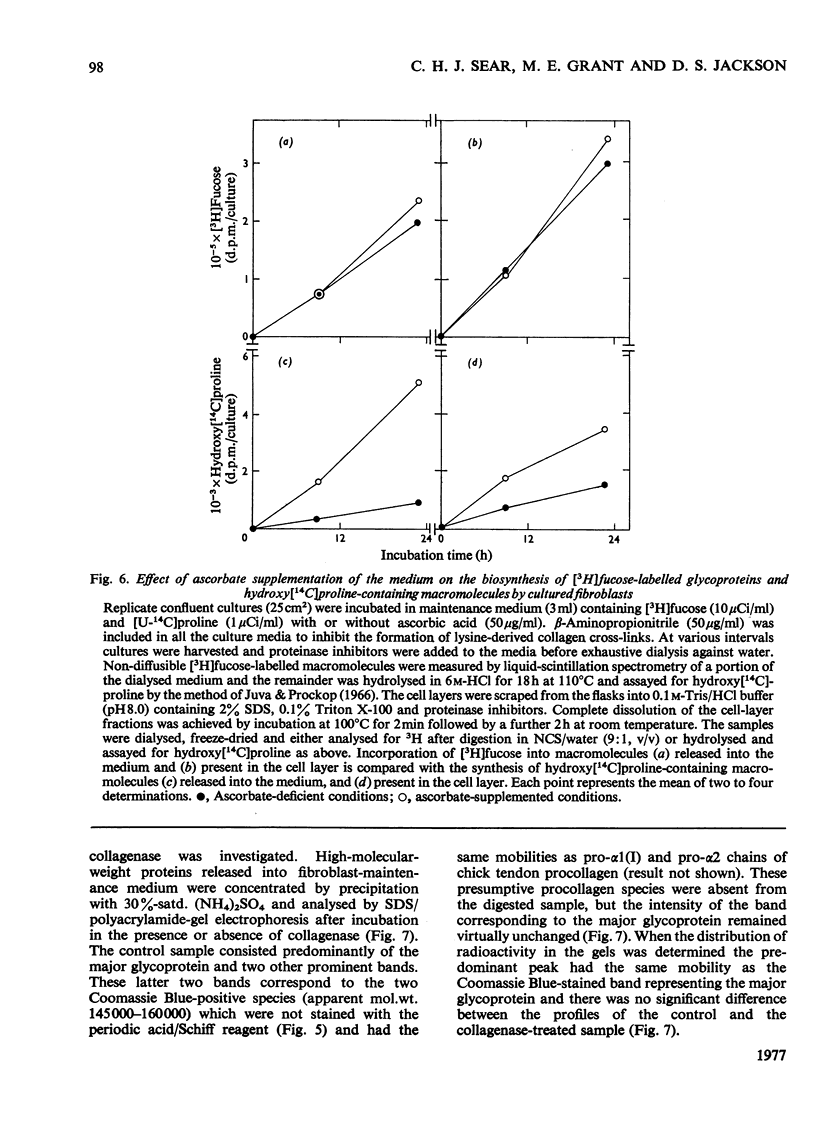
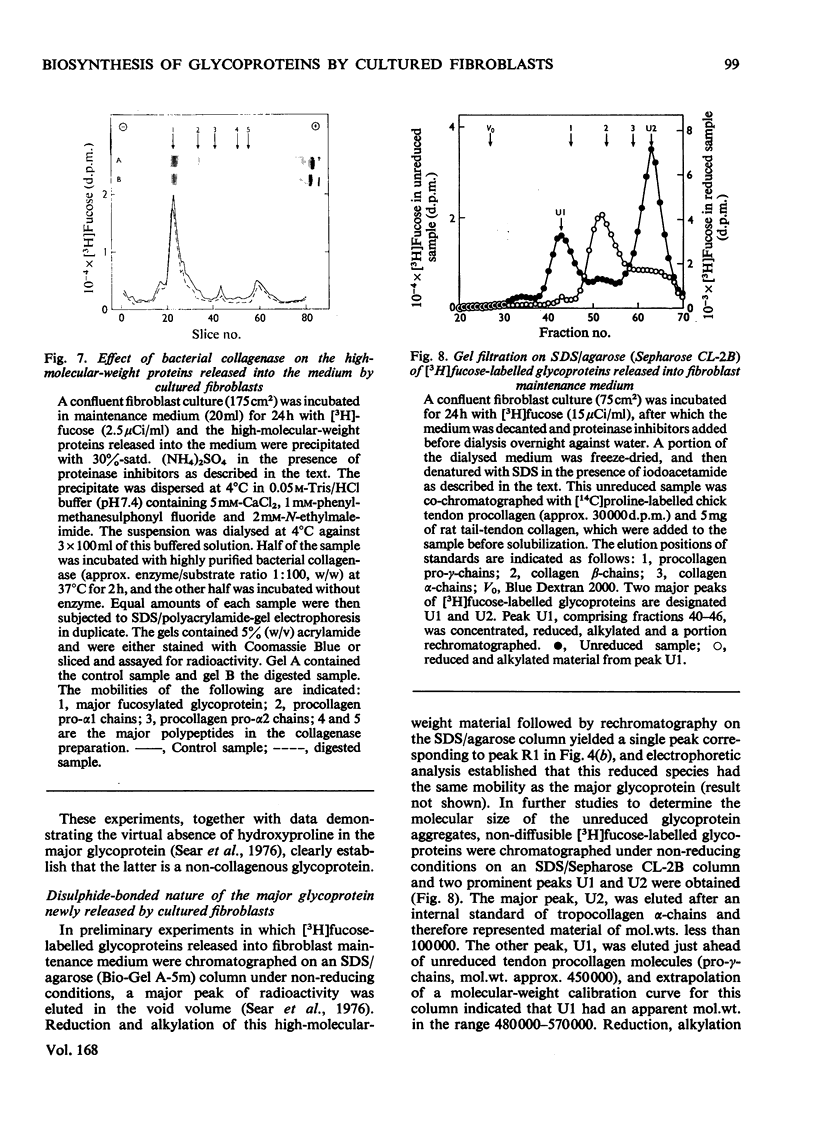



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. C. Glycoproteins of the connective tissue matrix. Int Rev Connect Tissue Res. 1976;7:251–322. doi: 10.1016/b978-0-12-363707-9.50012-5. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H. Synthesis and assembly of HeLa cell plasma membrane glycoproteins and proteins. J Biol Chem. 1975 Mar 25;250(6):2123–2134. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Barnes M. J. Function of ascorbic acid in collagen metabolism. Ann N Y Acad Sci. 1975 Sep 30;258:264–277. doi: 10.1111/j.1749-6632.1975.tb29287.x. [DOI] [PubMed] [Google Scholar]
- Bekesi J. G., Winzler R. J. The metabolism of plasma glycoproteins. Studies on the incorporation of L-fucose-1-14-C into tissue and serum in the normal rat. J Biol Chem. 1967 Sep 10;242(17):3873–3879. [PubMed] [Google Scholar]
- Bennett G., Leblond C. P. Formation of cell coat material for the whole surface of columnar cells in the rat small intestine, as visualized by radioautography with L-fucose-3H. J Cell Biol. 1970 Aug;46(2):409–416. doi: 10.1083/jcb.46.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COFFEY J. W., MILLER O. N., SELLINGER O. Z. THE METABOLISM OF L-FUCOSE IN THE RAT. J Biol Chem. 1964 Dec;239:4011–4017. [PubMed] [Google Scholar]
- Church R. L., Tanzer M. L. Isolation and amino acid composition of human procollagen [Pro alpha 1(I)]2 Pro alpha 2 from skin fibroblasts in culture. FEBS Lett. 1975 Apr 15;53(1):105–109. doi: 10.1016/0014-5793(75)80694-6. [DOI] [PubMed] [Google Scholar]
- Dell'Orco R. T., Mertens J. G., Kruse P. F., Jr Doubling potential, calendar time, and senescence of human diploid cells in culture. Exp Cell Res. 1973 Mar 15;77(1):356–360. doi: 10.1016/0014-4827(73)90588-0. [DOI] [PubMed] [Google Scholar]
- Dell'Orco R. T., Nash J. H., Kampschmidt R. F. Effects of ascorbic acid on collagen synthesis in nonmitotic human diploid fibroblasts. Proc Soc Exp Biol Med. 1973 Nov;144(2):621–622. doi: 10.3181/00379727-144-37647. [DOI] [PubMed] [Google Scholar]
- Della Corte E., Parkhouse R. M. Biosynthesis of immunoglobulin A (IgA). Secretion and addition of carbohydrate to monomer and polymer forms of a mouse myeloma protein. Biochem J. 1973 Nov;136(3):589–596. doi: 10.1042/bj1360589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson O., Ginsburg B. E., Hultberg B., Ockerman P. A. Influence of age and sex on plasma acid hydrolases. Clin Chim Acta. 1972 Aug;40(1):181–185. doi: 10.1016/0009-8981(72)90268-9. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GORHAM L. W., WAYMOUTH C. DIFFERENTIATION IN VITRO OF EMBRYONIC CARTILAGE AND BONE IN A CHEMICALLY-DEFINED MEDIUM. Proc Soc Exp Biol Med. 1965 May;119:287–290. doi: 10.3181/00379727-119-30160. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Epstein E. H., Jr, Sherr C. J. Precursors of collagen secreted by cultured human fibroblasts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3655–3659. doi: 10.1073/pnas.69.12.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. M., Hynes R. O., Davidson E. A., Bainton D. F. The location of proteins labeled by the 125I-lactoperoxidase system in the NIL 8 hamster fibroblast. Cell. 1975 Apr;4(4):353–365. doi: 10.1016/0092-8674(75)90156-7. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Herring G. M. A comparison of bone matrix and tendon with particular reference to glycoprotein content. Biochem J. 1976 Dec 1;159(3):749–755. doi: 10.1042/bj1590749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann H. Nonenzymatic tight binding of radioactivity to macromolecular fractions as a source of error in labeling experiments. Anal Biochem. 1974 May;59(1):293–301. doi: 10.1016/0003-2697(74)90036-0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Cell surface proteins and malignant transformation. Biochim Biophys Acta. 1976 Apr 30;458(1):73–107. doi: 10.1016/0304-419x(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Juva K., Prockop D. J. Modified procedure for the assay of H-3-or C-14-labeled hydroxyproline. Anal Biochem. 1966 Apr;15(1):77–83. doi: 10.1016/0003-2697(66)90249-1. [DOI] [PubMed] [Google Scholar]
- Kaufman R. L., Ginsburg V. The metabolism of L-fucose by HeLa cells. Exp Cell Res. 1968 Apr;50(1):127–132. doi: 10.1016/0014-4827(68)90400-x. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Dimeric character of fibronectin, a major cell surface-associated glycoprotein. Biochem Biophys Res Commun. 1977 Jan 24;74(2):699–706. doi: 10.1016/0006-291x(77)90359-x. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Silbert J. E., Silbert C. K. Heparan sulfate of skin fibroblasts grown in culture. Connect Tissue Res. 1975;4(1):17–23. doi: 10.3109/03008207509152193. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levene C. I., Bates C. J. Ascorbic acid and collagen synthesis in cultured fibroblasts. Ann N Y Acad Sci. 1975 Sep 30;258:288–306. doi: 10.1111/j.1749-6632.1975.tb29289.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein J. R., Byers P. H., Smith B. D., Martin G. R. Identification of the collagenous proteins synthesized by cultured cells from human skin. Biochemistry. 1975 Apr 22;14(8):1589–1594. doi: 10.1021/bi00679a007. [DOI] [PubMed] [Google Scholar]
- Linder E., Vaheri A., Ruoslahti E., Wartiovaara J. Distribution of fibroblast surface antigen in the developing chick embryo. J Exp Med. 1975 Jul 1;142(1):41–49. doi: 10.1084/jem.142.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalon R., Dorfman A. Acid mucopolysaccharides in cultured human fibroblasts. Lancet. 1969 Oct 18;2(7625):838–841. doi: 10.1016/s0140-6736(69)92289-2. [DOI] [PubMed] [Google Scholar]
- Mathews M. B. Comparative biochemistry of chondroitin sulphate-proteins of cartilage and notochord. Biochem J. 1971 Nov;125(1):37–46. doi: 10.1042/bj1250037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moczar M., Robert L. Action of human hyperlipemic sera on the biosynthesis of intercellular matrix macromolecules in aorta organ cultures. Paroi Arterielle. 1976 Sep;3(3):105–113. [PubMed] [Google Scholar]
- Muir L. W., Bornstein P., Ross R. A presumptive subunit of elastic fiber microfibrils secreted by arterial smooth-muscle cells in culture. Eur J Biochem. 1976 Apr 15;64(1):105–114. doi: 10.1111/j.1432-1033.1976.tb10278.x. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M. Immunoglobulin M biosynthesis. Production of intermediates and excess of light-chain in mouse myeloma MOPC 104E. Biochem J. 1971 Jul;123(4):635–641. doi: 10.1042/bj1230635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein E. Plasma membrane glycoprotein which mediates adhesion of fibroblasts to collagen. Nature. 1976 Aug 5;262(5568):497–500. doi: 10.1038/262497a0. [DOI] [PubMed] [Google Scholar]
- Schafer I. A., Silverman L., Sullivan J. C., Robertson W. V. Ascorbic acid deficiency in cultured human fibroblasts. J Cell Biol. 1967 Jul;34(1):83–95. doi: 10.1083/jcb.34.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
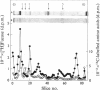
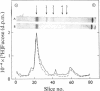
- Sear C. H., Grant M. E., Anderson J. C., Jackson D. S. The incorporation of L-[1-3-H]= fucose into non-collagenous glycoproteins secreted by human fibroblasts in culture. Biochem Soc Trans. 1975;3(1):138–140. doi: 10.1042/bst0030138. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Grant M. E., Jackson D. S. Identification of a major extracellular non-collagenous glycoprotein synthesised by human skin fibroblasts in culture. Biochem Biophys Res Commun. 1976 Jul 12;71(1):379–384. doi: 10.1016/0006-291x(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Sear C. H., Kewley M. A., Grant M. E., Steven F. S., Jackson D. S. Biosynthesis of a structural glycoprotein component of elastic tissues by cultured human skin fibroblasts. Biochem Soc Trans. 1977;5(2):430–431. doi: 10.1042/bst0050430. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Glycoproteins. Adv Protein Chem. 1973;27:349–467. doi: 10.1016/s0065-3233(08)60451-9. [DOI] [PubMed] [Google Scholar]
- Steiner S., Brennan P. J., Melnick J. L. Fucosylglycolipid metabolism in oncornavirus-transformed cell lines. Nat New Biol. 1973 Sep 5;245(140):19–21. doi: 10.1038/newbio245019a0. [DOI] [PubMed] [Google Scholar]
- Stewart M. L., Grollman A. P., Huang M. T. Aurintricarboxylic acid: inhibitor of initiation of protein synthesis. Proc Natl Acad Sci U S A. 1971 Jan;68(1):97–101. doi: 10.1073/pnas.68.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess J. M., Minaker E., Mitranic M. M., Moscarello M. A. The incorporation of L-fucose into glycoproteins in the Golgi apparatus of rat liver and in serum. Biochim Biophys Acta. 1973 Aug 17;320(1):123–132. doi: 10.1016/0304-4165(73)90172-4. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Goldberg B. The processing of procollagen in cultures of human and mouse fibroblasts. Arch Biochem Biophys. 1976 Apr;173(2):490–494. doi: 10.1016/0003-9861(76)90286-1. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E., Linder E., Wartiovaara J., Keski-Oja J., Kuusela P., Saksela O. Fibroblast surface antigen (SF): molecular properties, distribution in vitro and in vivo, and altered expression in transformed cells. J Supramol Struct. 1976;4(1):63–70. doi: 10.1002/jss.400040107. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weeds A. G., Hartley B. S. Selective purification of the thiol peptides of myosin. Biochem J. 1968 Apr;107(4):531–548. doi: 10.1042/bj1070531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Weston J. A. Isolation of a major cell surface glycoprotein from fibroblasts. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3492–3496. doi: 10.1073/pnas.71.9.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. Cell surface protein partially restores morphology, adhesiveness, and contact inhibition of movement to transformed fibroblasts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1217–1221. doi: 10.1073/pnas.73.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M., Yamada S. S., Pastan I. The major cell surface glycoprotein of chick embryo fibroblasts is an agglutinin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3158–3162. doi: 10.1073/pnas.72.8.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]