Abstract
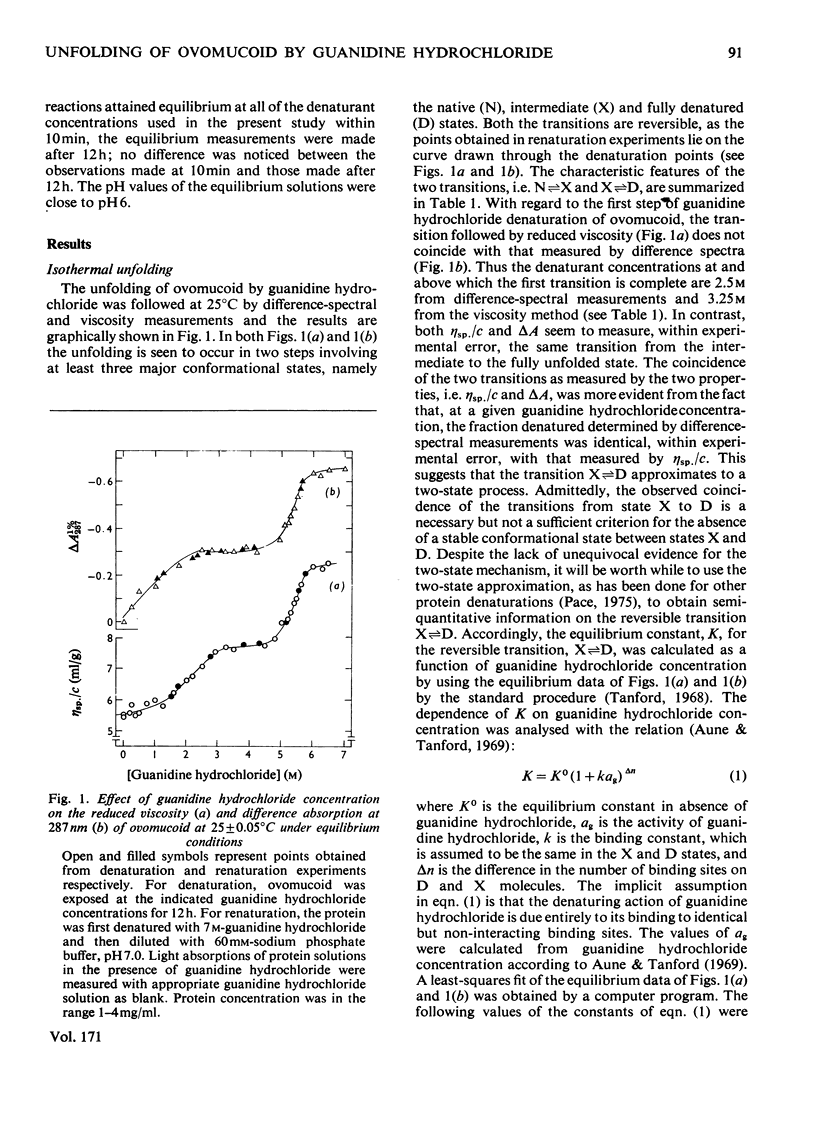
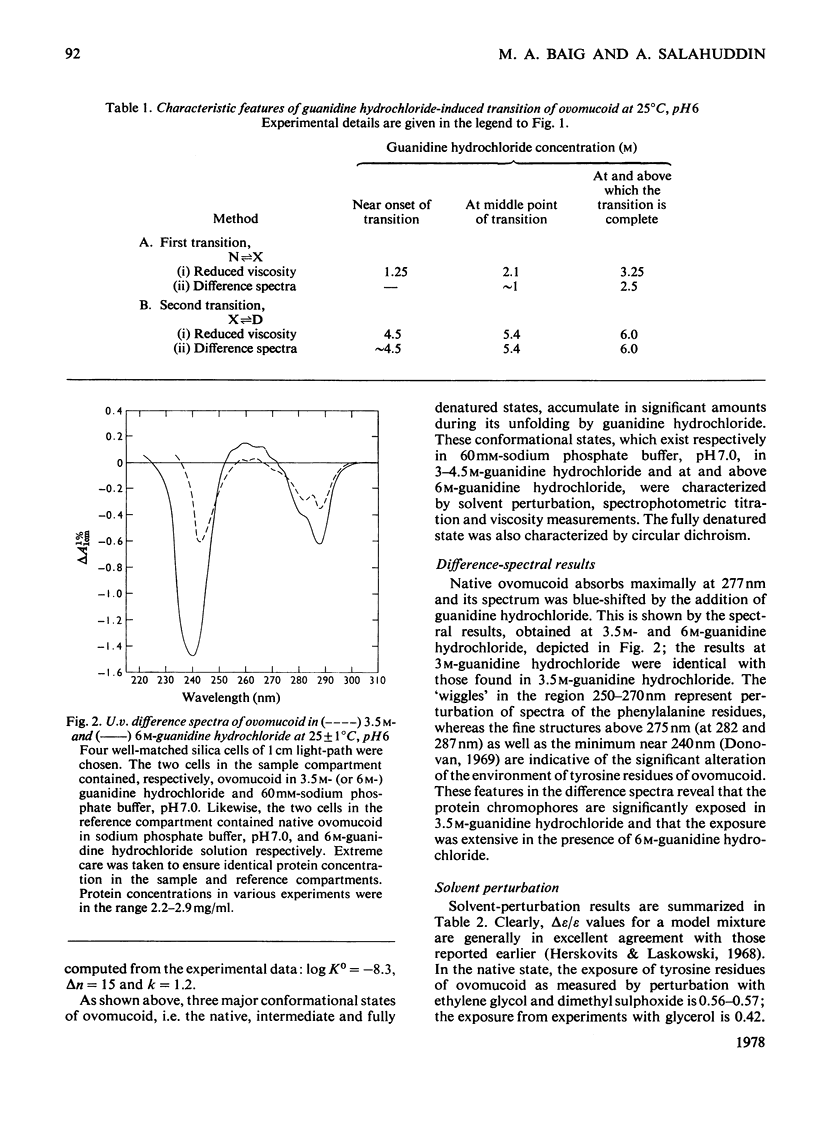
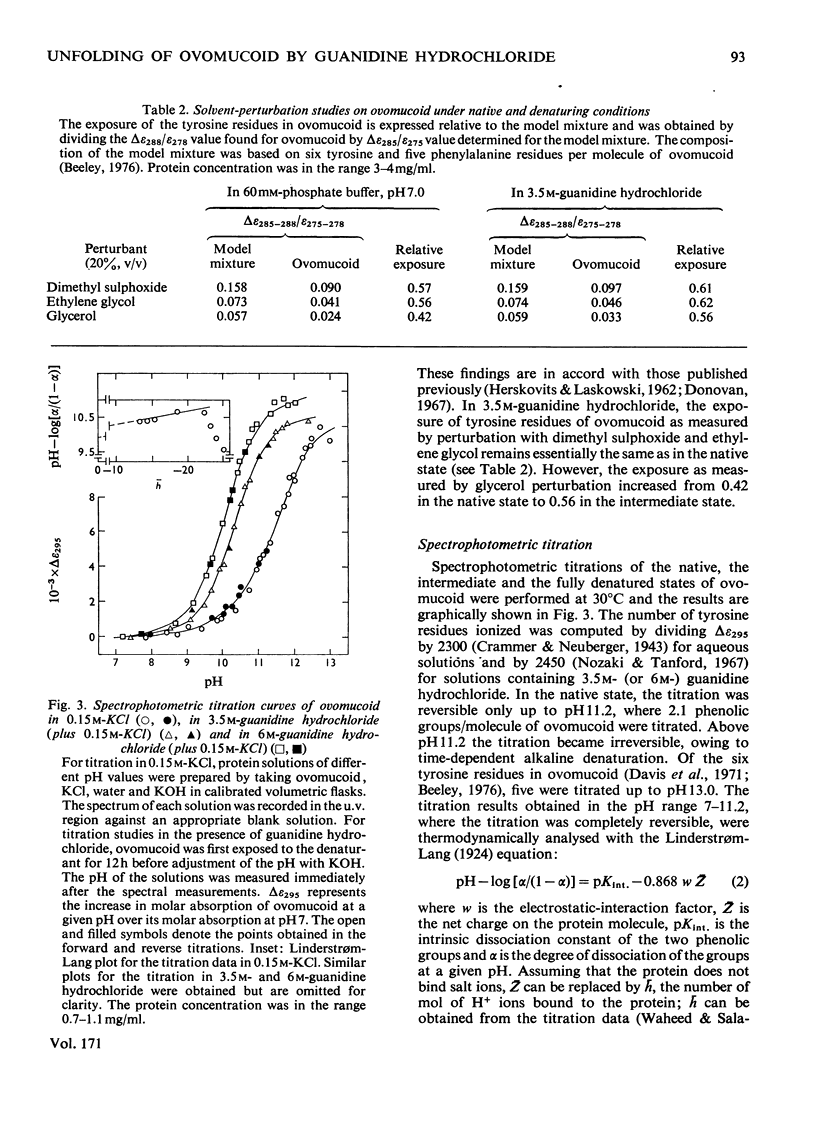
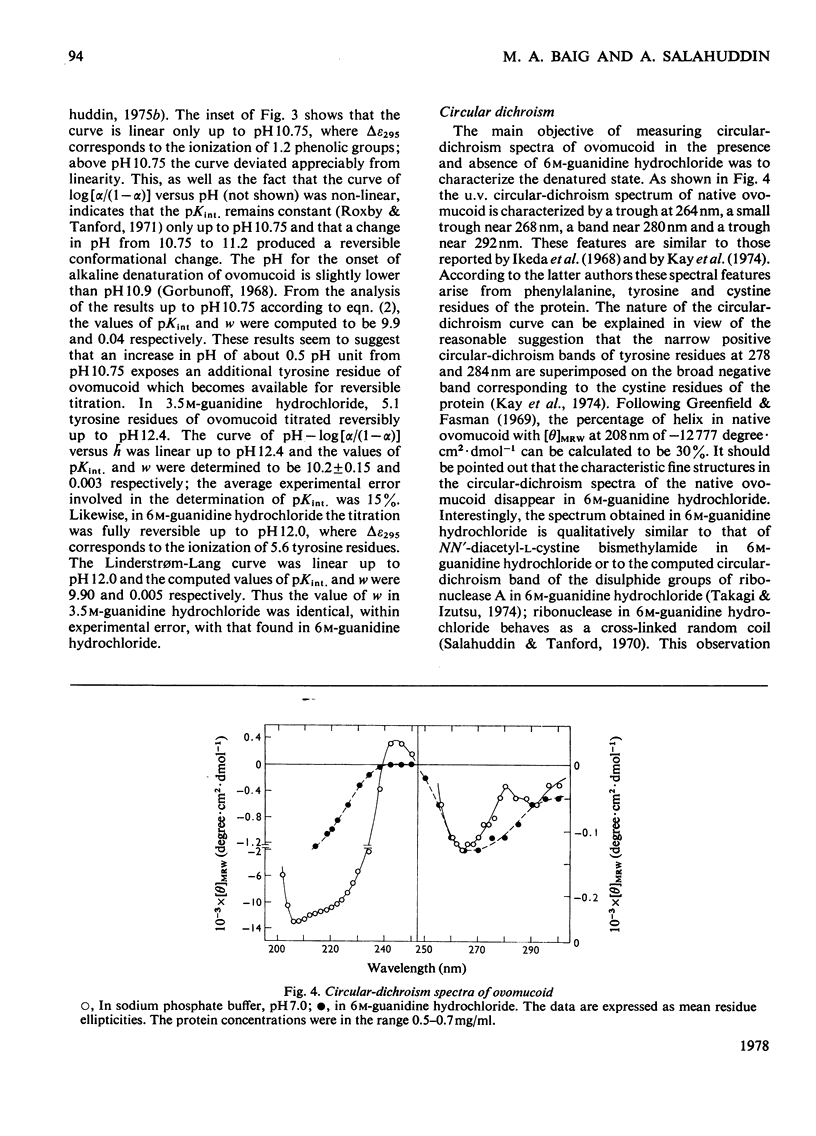
Reversible unfolding of ovomucoid by guanidine hydrochloride, as followed by viscosity and difference-spectral measurements at 25°C, pH6, occurred in two distinct steps involving at least three major conformational states, namely the native, intermediate and completely denatured states, occurring respectively in 60mm-sodium phosphate buffer, 3.5m-guanidine hydrochloride and 6m-guanidine hydrochloride. The overall native conformation of ovomucoid, as indicated by its intrinsic viscosity (5.24ml/g) and gel-filtration behaviour, differs significantly from that of a typical globular protein. Exposures of tyrosine residues in native ovomucoid measured by difference spectroscopy following perturbation with glycerol, ethylene glycol and dimethyl sulphoxide were, respectively, 0.42, 0.56 and 0.57. Of the exposed phenolic groups only one titrated normally (pKint., 9.91, electrostatic-interaction factor, w, 0.04). Results on difference spectra, solvent perturbation, phenolic titration and intrinsic viscosity (7.4ml/g) taken together showed that, although ovomucoid in 3.5m-guanidine hydrochloride was significantly unfolded, it retained a degree of native structure, removable with 6m-guanidine hydrochloride. In the latter, all the six tyrosine residues were available for titration, and the intrinsic viscosity of ovomucoid increased to 9.4ml/g. Furthermore, the characteristic fine structures in circular-dichrosim spectra of ovomucoid, associated with the elements of native structure, were abolished in 6m-guanidine hydrochloride, suggesting that the completely denatured state is structureless and presumably behaves as a cross-linked random coil. The latter state has been shown by analysis of the results on guanidine hydrochloride-dependence of the transition, intermediate⇌denatured, to be less stable than the intermediate state under native conditions by about 46kJ/mol at 25°C. Attempts have been made to interpret the above results in the light of available information on the amino acid sequence of ovomucoid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F., Salahuddin A. Influence of temperature on the intrinsic viscosities of proteins in random coil conformation. Biochemistry. 1974 Jan 15;13(2):245–249. doi: 10.1021/bi00699a003. [DOI] [PubMed] [Google Scholar]
- Ahmad F., Salahuddin A. Intrinsic viscosity of ovomucoid in random coil conformation. Int J Pept Protein Res. 1975;7(5):417–421. doi: 10.1111/j.1399-3011.1975.tb02462.x. [DOI] [PubMed] [Google Scholar]
- Ahmad F., Salahuddin A. Reversible unfolding of the major fraction of ovalbumin by guanidine hydrochloride. Biochemistry. 1976 Nov 16;15(23):5168–5175. doi: 10.1021/bi00668a034. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Ansari A. A., Kidwai S. A., Salahuddin A. Acetylation of amino groups and its effect on the conformation and immunological activity of obalbumin. J Biol Chem. 1975 Mar 10;250(5):1625–1632. [PubMed] [Google Scholar]
- Ansari A. A., Salahuddin A. Hydrogen-ion-titration curve of rabbit immunoglobulin against ovalbumin. Eur J Biochem. 1973 Jun;35(2):290–296. doi: 10.1111/j.1432-1033.1973.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Ansari A. A., Salahuddin A. Purification and some properties of rabbit anti-ovalbumin. Biochem J. 1973 Dec;135(4):705–711. doi: 10.1042/bj1350705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aune K. C., Tanford C. Thermodynamics of the denaturation of lysozyme by guanidine hydrochloride. II. Dependence on denaturant concentration at 25 degrees. Biochemistry. 1969 Nov;8(11):4586–4590. doi: 10.1021/bi00839a053. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Intermediates in protein folding reactions and the mechanism of protein folding. Annu Rev Biochem. 1975;44:453–475. doi: 10.1146/annurev.bi.44.070175.002321. [DOI] [PubMed] [Google Scholar]
- Beeley J. G. Active fragments obtained by cyanogen bromide cleavage of ovomucoid. Biochem J. 1976 May 1;155(2):345–351. doi: 10.1042/bj1550345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chignell D. A., Azhir A., Gratzer W. B. The denaturation of muscle phosphorylase b by urea. Eur J Biochem. 1972 Mar 15;26(1):37–42. doi: 10.1111/j.1432-1033.1972.tb01736.x. [DOI] [PubMed] [Google Scholar]
- Crammer J. L., Neuberger A. The state of tyrosine in egg albumin and in insulin as determined by spectrophotometric titration. Biochem J. 1943 Jul;37(2):302–310. doi: 10.1042/bj0370302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. G., Mapes C. J., Donovan J. W. Batch purification of ovomucoid and characterization of the purified product. Biochemistry. 1971 Jan 5;10(1):39–42. doi: 10.1021/bi00777a006. [DOI] [PubMed] [Google Scholar]
- Donovan J. W. A spectrophotometric and spectrofluorometric study of intramolecular interactions of phenolic groups in ovomucoid. Biochemistry. 1967 Dec;6(12):3918–3927. doi: 10.1021/bi00864a038. [DOI] [PubMed] [Google Scholar]
- Donovan J. W. Changes in ultraviolet absorption produced by alteration of protein conformation. J Biol Chem. 1969 Apr 25;244(8):1961–1967. [PubMed] [Google Scholar]
- Gorbunoff M. J. Exposure of tyrosine residues in proteins. II. The reactions of cyanuric fluoride and N-acetylimidazole with pepsinogen, soybean trypsin inhibitor, and ovomucoid. Biochemistry. 1968 Jul;7(7):2547–2554. doi: 10.1021/bi00847a015. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- HERSKOVITS T. T., LASKOWSKI M., Jr Location of chromophoric residues in proteins by solvent perturbation. II. Tyrosyls in ovomucoid. J Biol Chem. 1962 Nov;237:3418–3422. [PubMed] [Google Scholar]
- Herskovits T. T., Laskowski M., Jr Location of chromophoric residues in proteins by solvent perturbation. IV. Tyrosyl residues in ribonuclease. J Biol Chem. 1968 May 10;243(9):2123–2129. [PubMed] [Google Scholar]
- Ikeda K., Hamaguchi K., Yamamoto M., Ikenaka T. Circular dichroism and optical rotatory dispersion of trypsin inhibitors. J Biochem. 1968 Apr;63(4):521–531. doi: 10.1093/oxfordjournals.jbchem.a128806. [DOI] [PubMed] [Google Scholar]
- Kay E., Strickland E. H., Billups C. Near ultraviolet circular dichroism and absorption spectra of chicken ovomucoid and acetylated derivatives at 297 and 77 degrees K. J Biol Chem. 1974 Feb 10;249(3):797–802. [PubMed] [Google Scholar]
- Laskowski M., Jr Measurement of accessibility of protein chromophores by solvent perturbation of their ultraviolet spectra. Fed Proc. 1966 Jan-Feb;25(1):20–27. [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. Acid-base titrations in concentrated guanidine hydrochloride. Dissociation constants of the guamidinium ion and of some amino acids. J Am Chem Soc. 1967 Feb 15;89(4):736–742. doi: 10.1021/ja00980a002. [DOI] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- Pace C. N. The stability of globular proteins. CRC Crit Rev Biochem. 1975 May;3(1):1–43. doi: 10.3109/10409237509102551. [DOI] [PubMed] [Google Scholar]
- Qasim M. A., Salahuddin A. Iionization of tyrosyl groups of ovalbumin under native and denaturing conditions. Biochim Biophys Acta. 1977 Feb 22;490(2):515–522. doi: 10.1016/0005-2795(77)90028-9. [DOI] [PubMed] [Google Scholar]
- Riddiford L. M. Solvent perturbation and ultraviolet optical rotatory dispersion studies of paramyosin. J Biol Chem. 1966 Jun 25;241(12):2792–2802. [PubMed] [Google Scholar]
- Roxby R., Tanford C. Hydrogen ion titration curve of lysozyme in 6 M guanidine hydrochloride. Biochemistry. 1971 Aug 31;10(18):3348–3352. doi: 10.1021/bi00794a005. [DOI] [PubMed] [Google Scholar]
- Salahuddin A., Tanford C. Thermodynamics of the denaturation of ribonuclease by guanidine hydrochloride. Biochemistry. 1970 Mar 17;9(6):1342–1347. doi: 10.1021/bi00808a007. [DOI] [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Waheed A., Salahuddin A. Hydrogen-ion titration curve of ovomucoid. Biochim Biophys Acta. 1975 Jan 30;379(1):147–156. doi: 10.1016/0005-2795(75)90016-1. [DOI] [PubMed] [Google Scholar]
- Waheed A., Salhuddin A. Isolation and characterization of a variant of ovomucoid. Biochem J. 1975 Apr;147(1):139–144. doi: 10.1042/bj1470139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. P., Tanford C. Denaturation of bovine carbonic anhydrase B by guanidine hydrochloride. A process involving separable sequential conformational transitions. J Biol Chem. 1973 Dec 25;248(24):8518–8523. [PubMed] [Google Scholar]
- Ziegler S. M., Bush C. A. Circular dichroism of cyclic hexapeptides with one and two side chains. Biochemistry. 1971 Apr 13;10(8):1330–1335. doi: 10.1021/bi00784a009. [DOI] [PubMed] [Google Scholar]


