Abstract
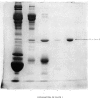
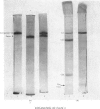
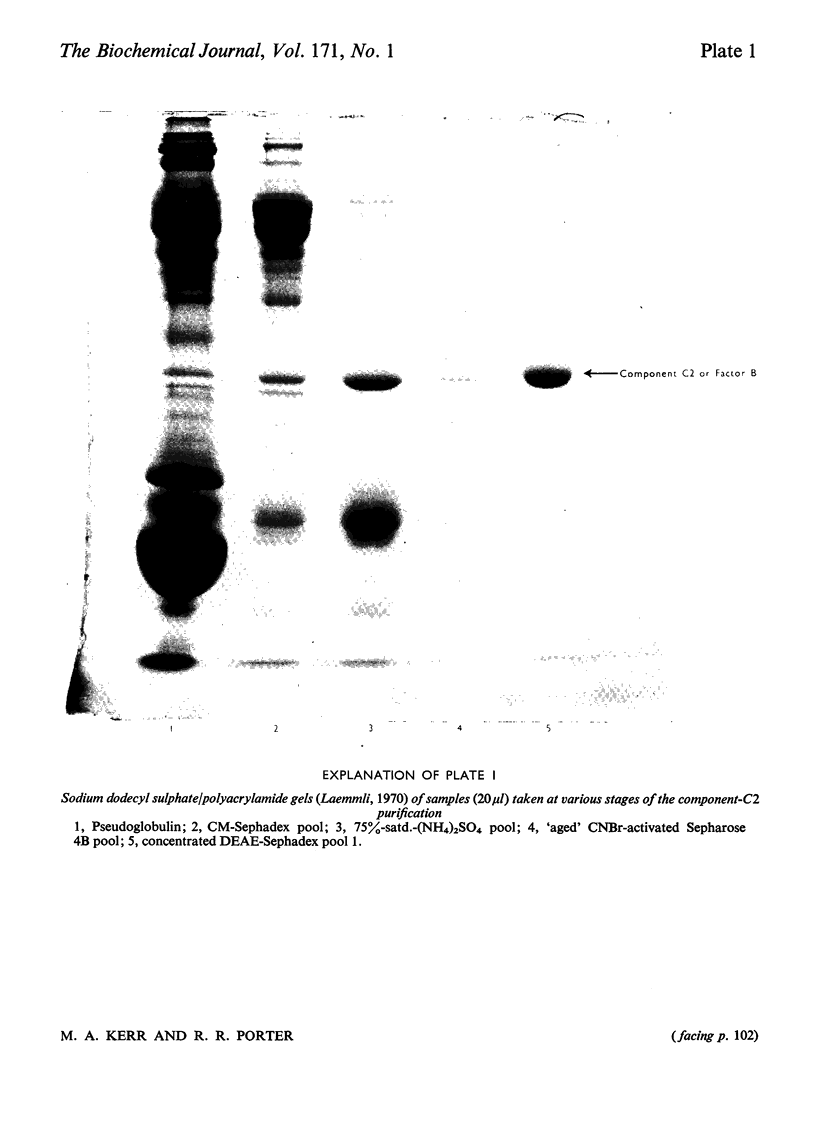
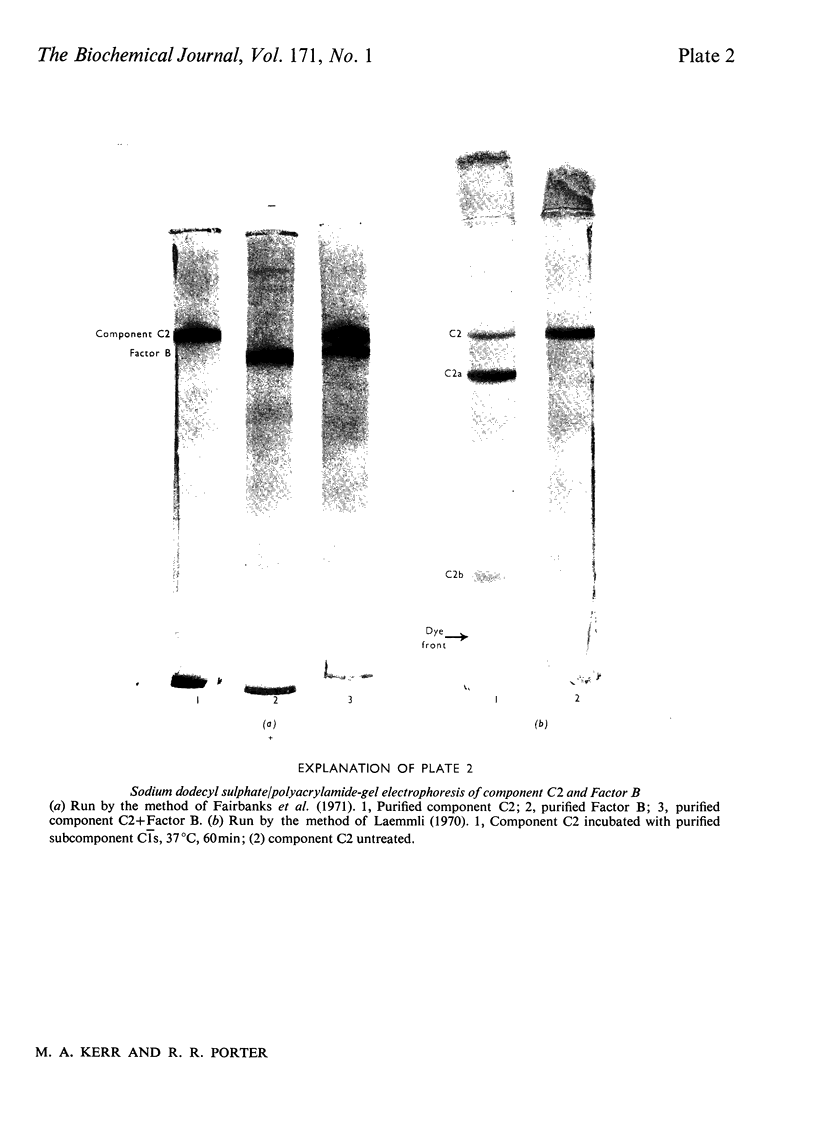
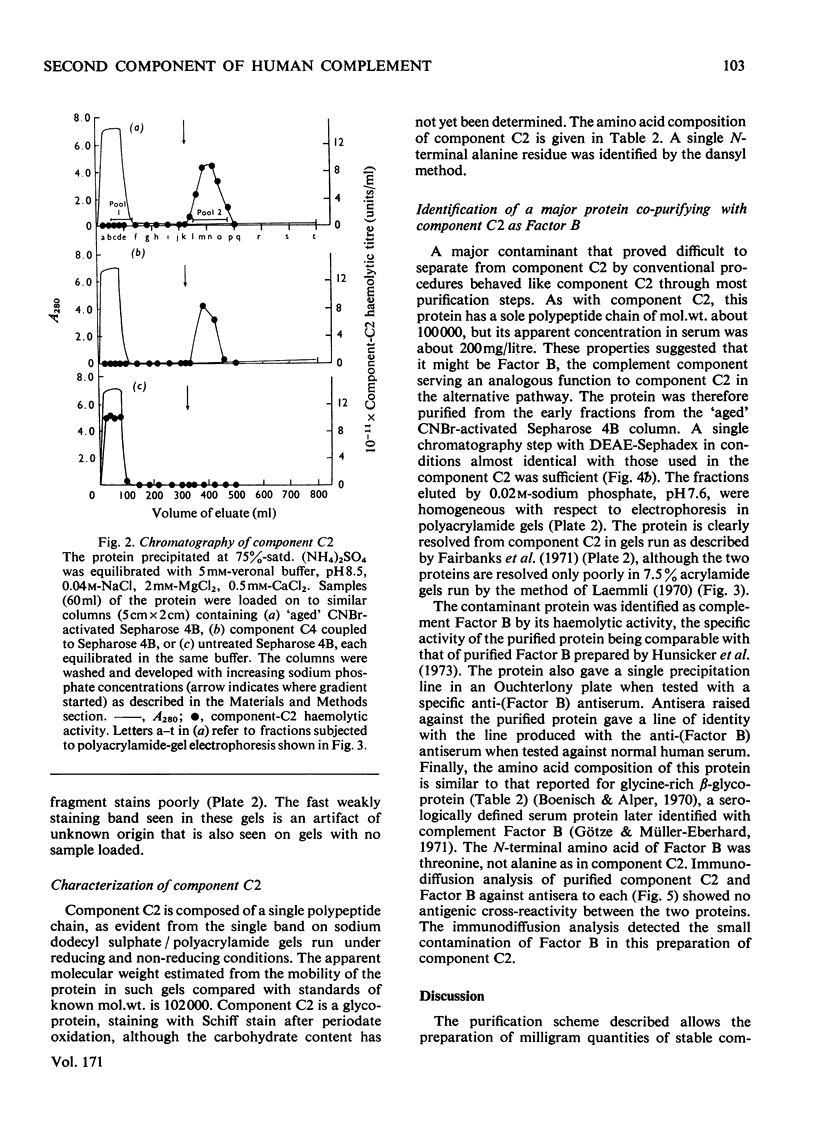
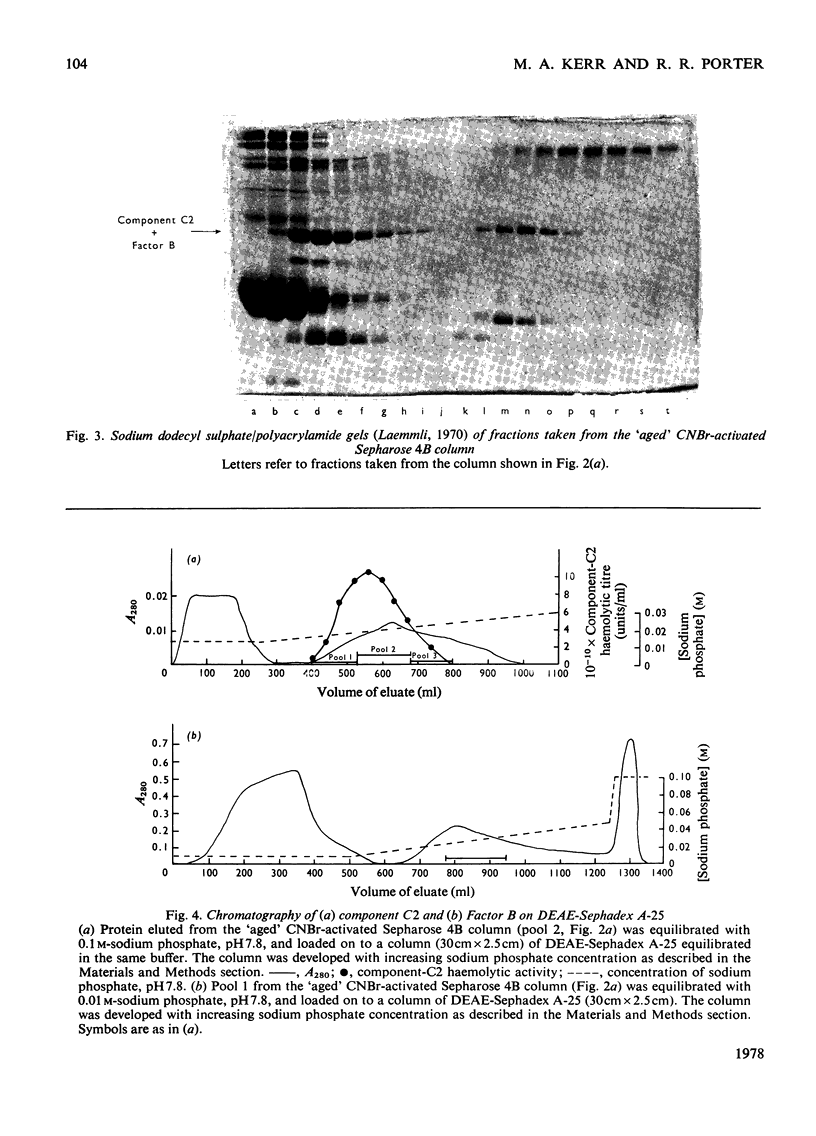
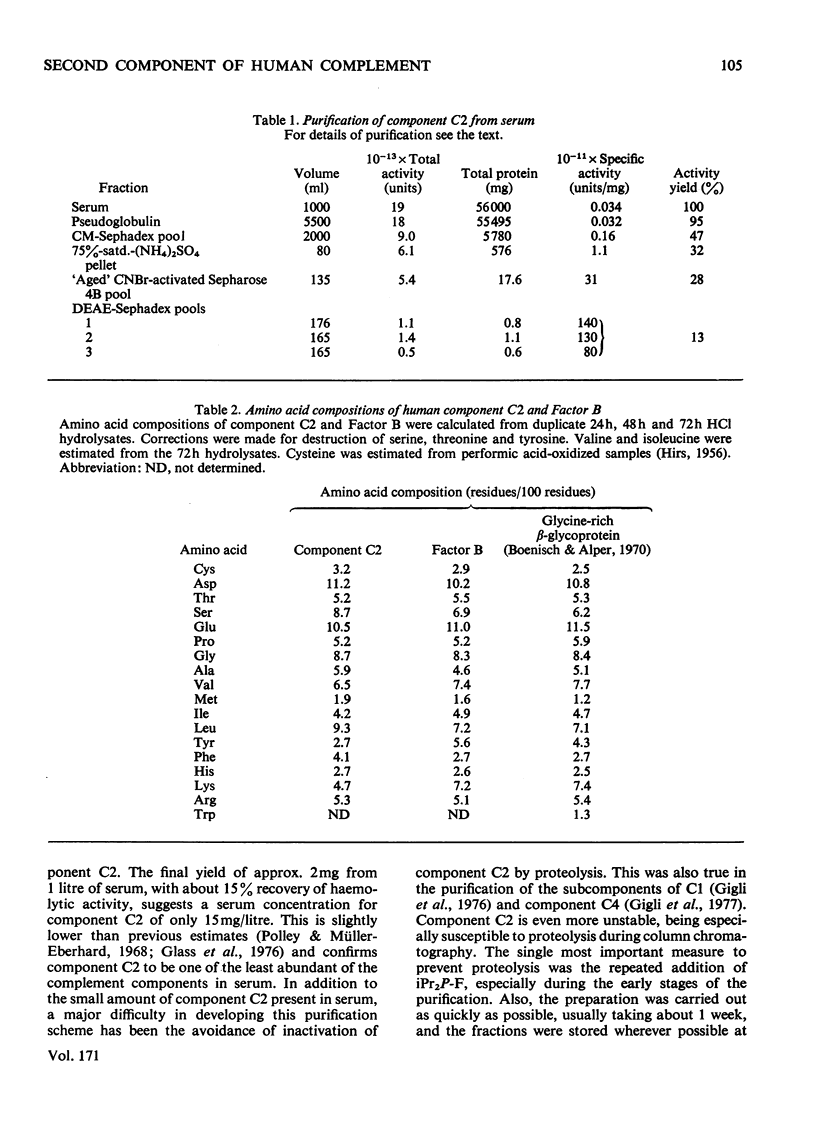
The second component of human complement (C2) was purified by a combination of euglobulin precipitation, ion-exchange chromatography, (NH4)2SO4 precipitation and affinity chromatography. The final product was homogeneous by the criterion of polyacrylamide-gel electrophoresis and represents a purification of about 4000-fold from serum with 15-20% yield. Component C2 comprises a single carbohydrate-containing polypeptide chain, with an apparent mol.wt. of 102000; alanine is the N-terminal amino acid. The molecule is rapidly cleaved by activated subcomponent C1s with the loss of haemolytic activity to yield two fragments with apparent mol.wts. of 74000 and 34000. These fragments are not linked by disulphide bonds and can be easily separated. A second protein isolated during the purification of component C2 was identified by its haemolytic and antigenic properties as complement Factor B, the protein serving an analogous function to component C2 in the alternative pathway. The protein, which is also a single carbohydrate-containing polypeptide chain, has an apparent mol.wt. of 95000 and threonine as N-terminal amino acid. The amino acid analyses of component C2 and Factor B are compared.
Full text
PDF


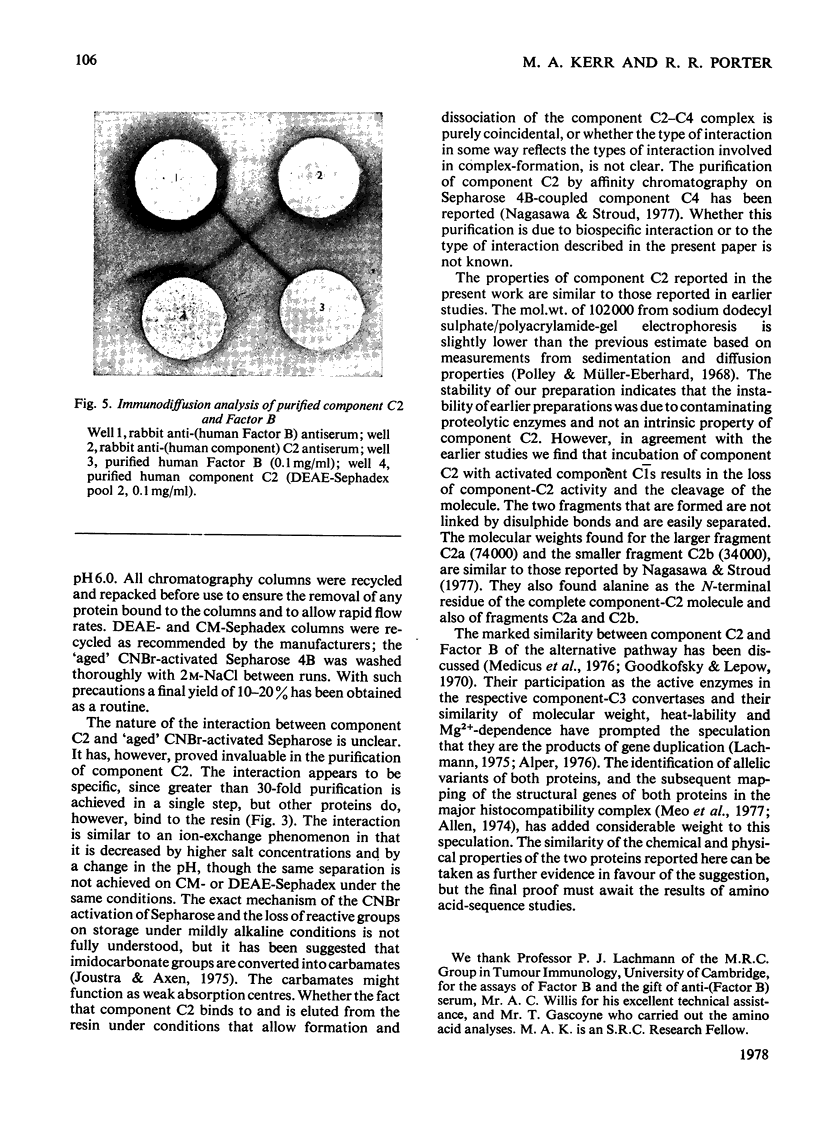

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen F. H., Jr Linkage of HL-A and GBG. Vox Sang. 1974;27(4):382–384. doi: 10.1111/j.1423-0410.1974.tb02433.x. [DOI] [PubMed] [Google Scholar]
- Alper C. A. Inherited structural polymorphism in human C2: evidence for genetic linkage between C2 and Bf. J Exp Med. 1976 Oct 1;144(4):1111–1115. doi: 10.1084/jem.144.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenisch T., Alper C. A. Isolation and properties of a glycine-rich beta-glycoprotein of human serum. Biochim Biophys Acta. 1970 Dec 22;221(3):529–535. doi: 10.1016/0005-2795(70)90224-2. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J., Colten H. R. Immune hemolysis and the functional properties of the second (C2) and fourth (C4) components of complement. I. Functional differences among C4 sites on cell surfaces. J Immunol. 1970 Dec;105(6):1439–1446. [PubMed] [Google Scholar]
- Cooper N. R. Enzymatic activity of the second component of complement. Biochemistry. 1975 Sep 23;14(19):4245–4251. doi: 10.1021/bi00690a015. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fu S. M., Kunkel H. G., Brusman H. P., Allen F. H., Jr, Fotino M. Evidence for linkage between HL-A histocompatibility genes and those involved in the synthesis of the second component of complement. J Exp Med. 1974 Oct 1;140(4):1108–1111. doi: 10.1084/jem.140.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., Porter R. R., Sim R. B. The unactivated form of the first component of human complement, C1. Biochem J. 1976 Sep 1;157(3):541–548. doi: 10.1042/bj1570541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigli I., von Zabern I., Porter R. R. The isolation and structure of C4, the fourth component of human complement. Biochem J. 1977 Sep 1;165(3):439–446. doi: 10.1042/bj1650439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass D., Raum D., Gibson D., Stillman J. S., Schur P. H. Inherited deficiency of the second component of complement. Rheumatic disease associations. J Clin Invest. 1976 Oct;58(4):853–861. doi: 10.1172/JCI108538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodkofsky I., Lepow I. H. Functional relationship of factor B in the properdin system to C3 proactivator of human serum. J Immunol. 1971 Oct;107(4):1200–1204. [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The alternative pathway of complement activation. Adv Immunol. 1976;24:1–35. doi: 10.1016/s0065-2776(08)60328-4. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Hunsicker L. G., Ruddy S., Austen K. F. Alternate complement pathway: factors involved in cobra venom factor (CoVF) activation of the third component of complement (C3). J Immunol. 1973 Jan;110(1):128–138. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin A., Lachmann P. J., Halbwachs L., Hobart M. J. Haemolytic diffusion plate assays for factors B and D of the alternative pathway of complement activation. Immunochemistry. 1976 Apr;13(4):317–324. doi: 10.1016/0019-2791(76)90341-4. [DOI] [PubMed] [Google Scholar]
- Mayer M. M., Miller J. A., Shin H. S. A specific method for purification of the second component of guinea pig complement and a chemical evaluation of the one-hit theory. J Immunol. 1970 Aug;105(2):327–341. [PubMed] [Google Scholar]
- Medicus R. G., Götze O., Müller-Eberhard H. J. The serine protease nature of the C3 and C5 convertases of the classical and alternative complement pathways. Scand J Immunol. 1976;5(9):1049–1055. doi: 10.1111/j.1365-3083.1976.tb03056.x. [DOI] [PubMed] [Google Scholar]
- Meo T., Atkinson J. P., Bernoco M., Bernoco D., Ceppellini R. Structural heterogeneity of C2 Complement protein and its genetic variants in man: a new polymorphism of the HLA region. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1672–1675. doi: 10.1073/pnas.74.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Müller-Eberhard H. J. The second component of human complement: its isolation, fragmentation by C'1 esterase, and incorporation into C'3 convertase. J Exp Med. 1968 Sep 1;128(3):533–551. doi: 10.1084/jem.128.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid K. B. A collagen-like amino acid sequence in a polypeptide chain of human C1q (a subcomponent of the first component of complement). Biochem J. 1974 Jul;141(1):189–203. doi: 10.1042/bj1410189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]