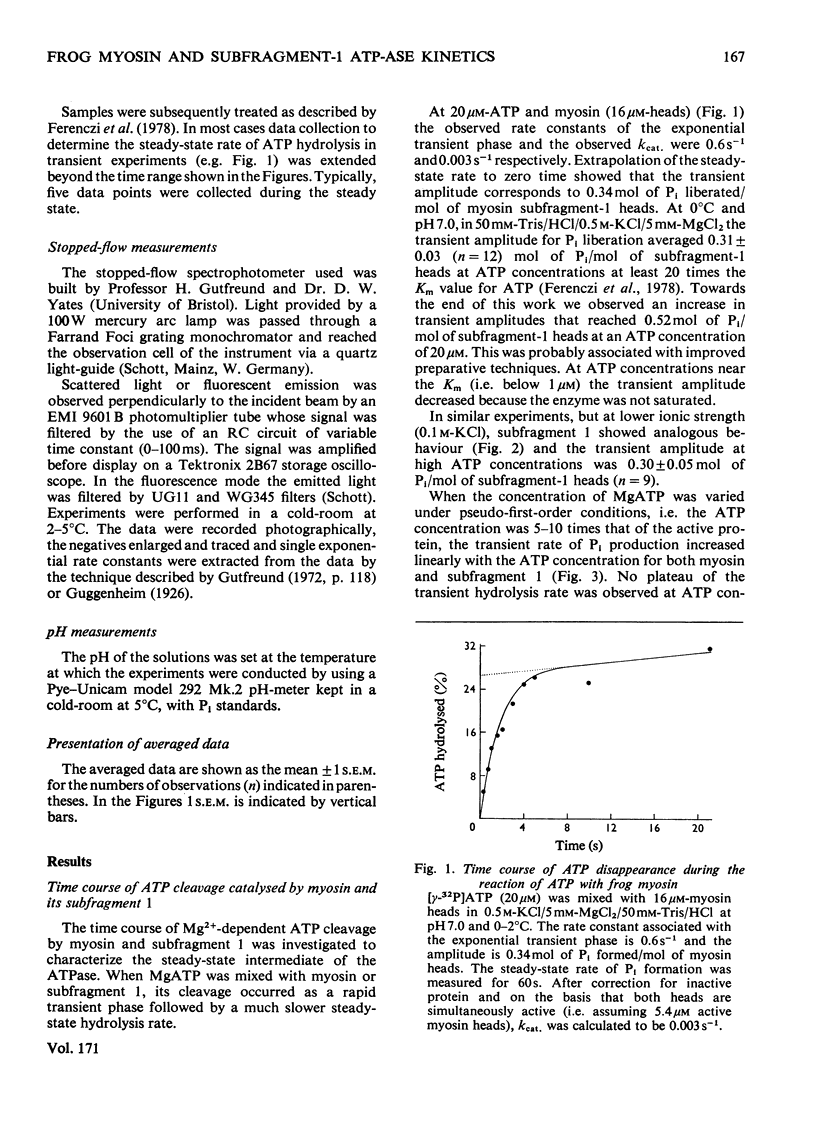
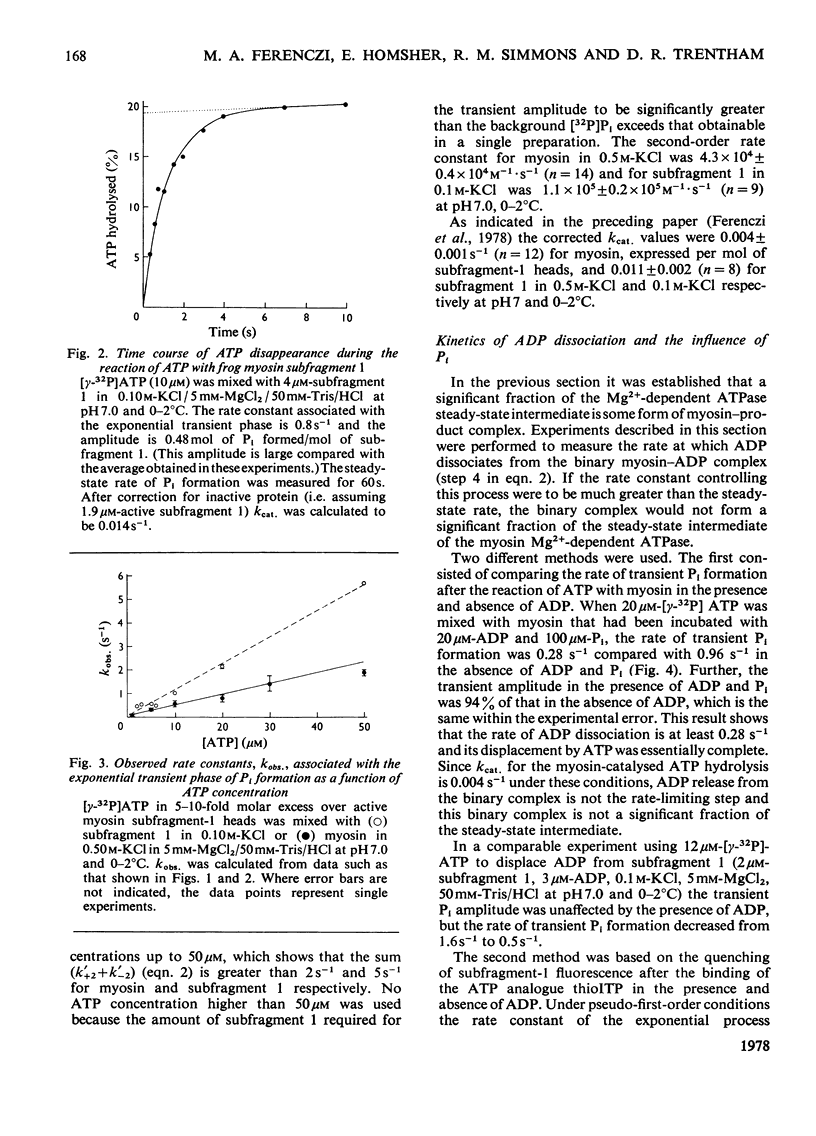
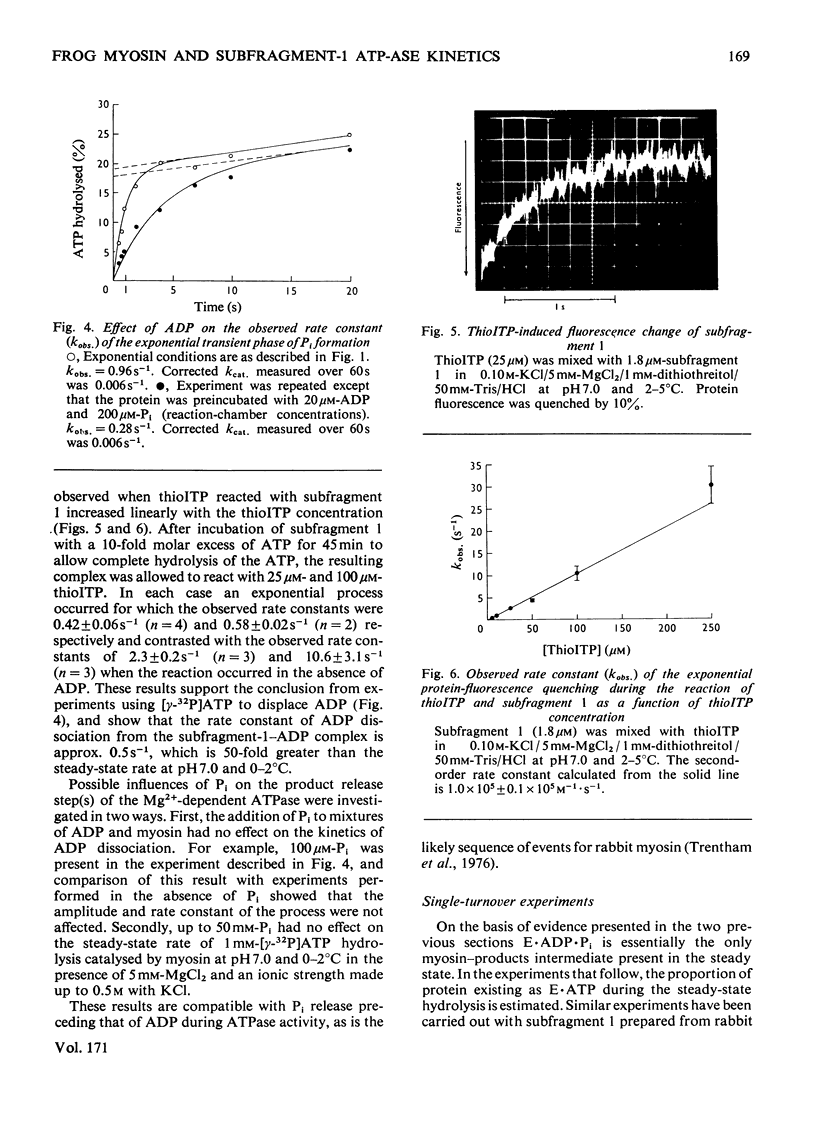
Abstract
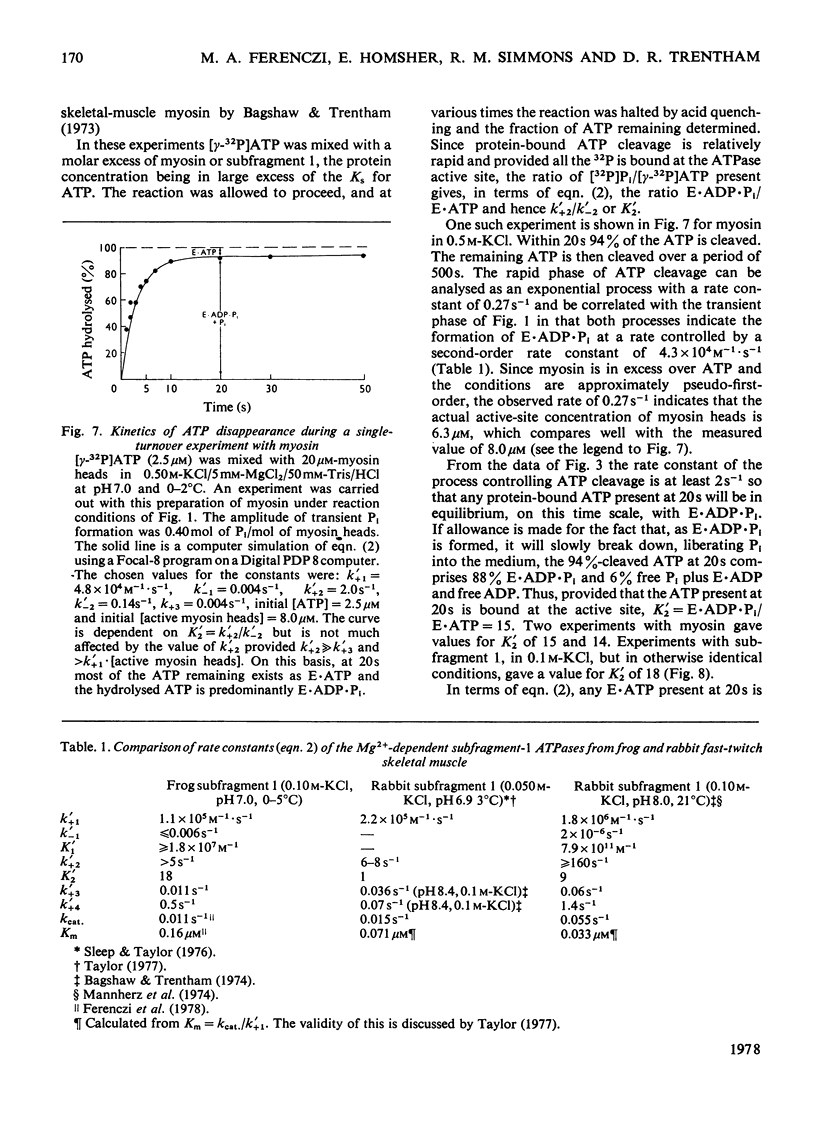
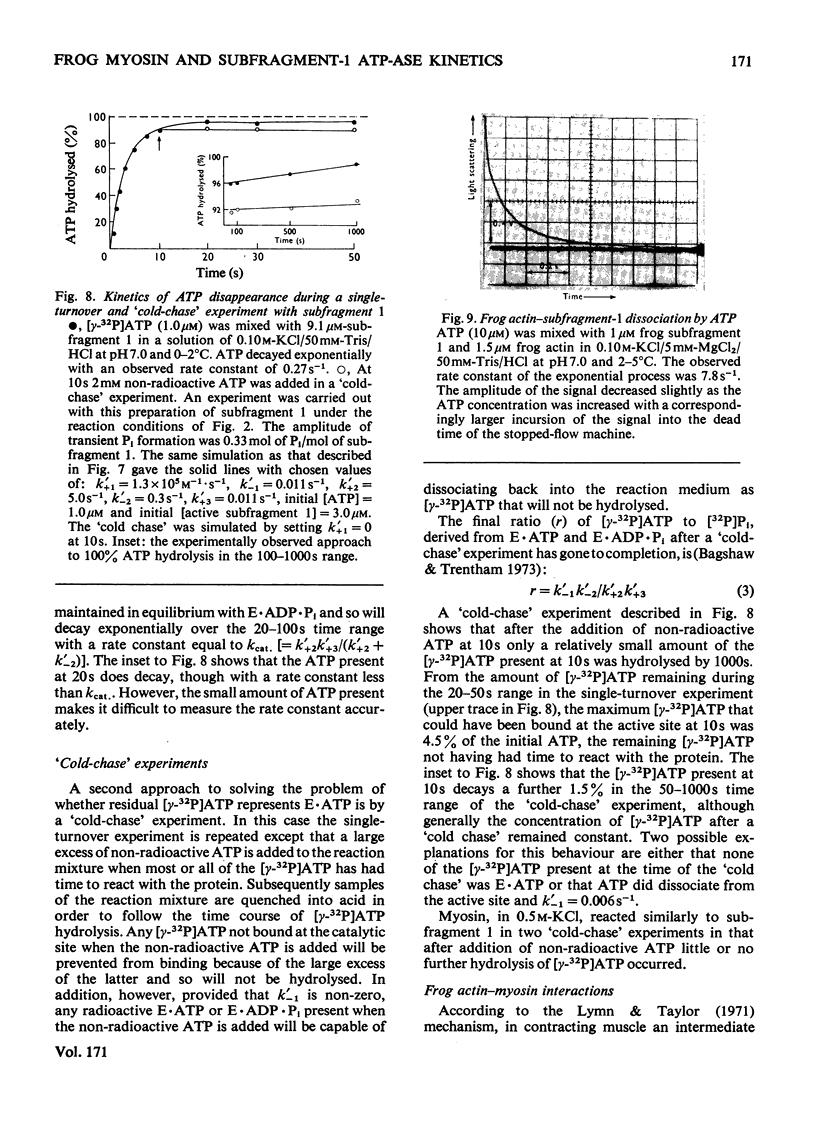
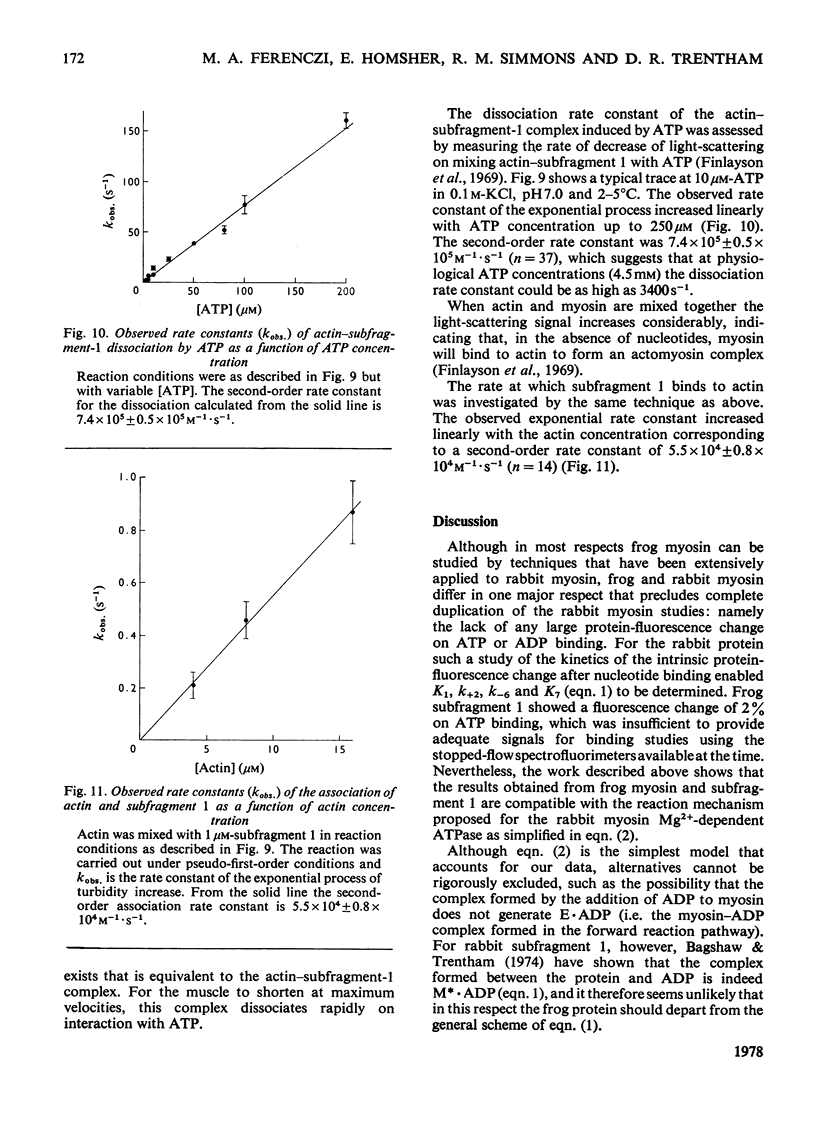
The Mg2+-dependent ATPase (adenosine 5'-triphosphatase) mechanism of myosin and subfragment 1 prepared from frog leg muscle was investigated by transient kinetic technique. The results show that in general terms the mechanism is similar to that of the rabbit skeletal-muscle myosin ATPase. During subfragment-1 ATPase activity at 0-5 degrees C pH 7.0 and I0.15, the predominant component of the steady-state intermediate is a subfragment-1-products complex (E.ADP.Pi). Binary subfragment-1-ATP (E.ATP) and subfragment-1-ADP (E.ADP) complexes are the other main components of the steady-state intermediate, the relative concentrations of the three components E.ATP, E.ADP.Pi and E.ADP being 5.5:92.5:2.0 respectively. The frog myosin ATPase mechanism is distinguished from that of the rabbit at 0-5 degrees C by the low steady-state concentrations of E.ATP and E.ADP relative to that of E.ADP.Pi and can be described by: E + ATP k' + 1 in equilibrium k' - 1 E.ATP k' + 2 in equilibrium k' - 2 E.ADP.Pi k' + 3 in equilibrium k' - 3 E.ADP + Pi k' + 4 in equilibrium k' - 4 E + ADP. In the above conditions successive forward rate constants have values: k' + 1, 1.1 X 10(5)M-1.S-1; k' + 2 greater than 5s-1; k' + 3, 0.011 s-1; k' + 4, 0.5 s-1; k'-1 is probably less than 0.006s-1. The observed second-order rate constants of the association of actin to subfragment 1 and of ATP-induced dissociation of the actin-subfragment-1 complex are 5.5 X 10(4) M-1.S-1 and 7.4 X 10(5) M-1.S-1 respectively at 2-5 degrees C and pH 7.0. The physiological implications of these results are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagshaw C. R., Eccleston J. F., Eckstein F., Goody R. S., Gutfreund H., Trentham D. R. The magnesium ion-dependent adenosine triphosphatase of myosin. Two-step processes of adenosine triphosphate association and adenosine diphosphate dissociation. Biochem J. 1974 Aug;141(2):351–364. doi: 10.1042/bj1410351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin N. A., Gilbert C., Kretzschmar K. M., Wilkie D. R. The effect of the performance of work on total energy output and metabolism during muscular contraction. J Physiol. 1974 May;238(3):455–472. doi: 10.1113/jphysiol.1974.sp010537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Ferenczi M. A., Homsher E., Trentham D. R., Weeds A. G. Preparation and characterization of frog muscle myosin subfragment 1 and actin. Biochem J. 1978 Apr 1;171(1):155–163. doi: 10.1042/bj1710155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson B., Lymn R. W., Taylor E. W. Studies on the kinetics of formation and dissociation of the actomyosin complex. Biochemistry. 1969 Mar;8(3):811–819. doi: 10.1021/bi00831a008. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Paul R. J. Aerobic recovery metabolism following a single isometric tetanus in frog sartorius muscle at 0 degrees C. J Physiol. 1976 Jan;254(3):693–709. doi: 10.1113/jphysiol.1976.sp011253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. M., Umazume Y., Kushmerick M. J. Ca2+ dependence of tension and ADP production in segments of chemically skinned muscle fibers. Biochim Biophys Acta. 1976 May 14;430(2):352–365. doi: 10.1016/0005-2728(76)90091-8. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Transient state phosphate production in the hydrolysis of nucleoside triphosphates by myosin. Biochemistry. 1970 Jul 21;9(15):2975–2983. doi: 10.1021/bi00817a007. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Schenck H., Goody R. S. Synthesis of ATP from ADP and inorganic phosphate at the myosin-subfragment 1 active site. Eur J Biochem. 1974 Oct 1;48(1):287–295. doi: 10.1111/j.1432-1033.1974.tb03767.x. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Tregear R. T. Evidence for a complex between myosin and ADP in relaxed muscle fibres. Nat New Biol. 1972 Jan 5;235(53):23–24. doi: 10.1038/newbio235023a0. [DOI] [PubMed] [Google Scholar]
- Marston S. The nucleotide complexes of myosin in glycerol-extracted muscle fibres. Biochim Biophys Acta. 1973 May 30;305(2):397–412. doi: 10.1016/0005-2728(73)90186-2. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Taylor E. W. Intermediate states of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5813–5817. doi: 10.1021/bi00671a019. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Transient phase of adenosine triphosphate hydrolysis by myosin, heavy meromyosin, and subfragment 1. Biochemistry. 1977 Feb 22;16(4):732–739. doi: 10.1021/bi00623a027. [DOI] [PubMed] [Google Scholar]
- Taylor R. S., Weeds A. G. The magnesium-ion-dependent adenosine triphosphatase of bovine cardiac Myosin and its subfragment-1. Biochem J. 1976 Nov;159(2):301–315. doi: 10.1042/bj1590301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. S., Weeds A. G. Transient-phase of ATP hydrolysis by myosin sub-fragment-1 isoenzymes. FEBS Lett. 1977 Mar 15;75(1):55–60. doi: 10.1016/0014-5793(77)80052-5. [DOI] [PubMed] [Google Scholar]
- Tonomura Y., Inoue A. The substructure of myosin and the reaction mechanism of its adenosine triphosphatase. Mol Cell Biochem. 1974 Dec 20;5(3):127–143. doi: 10.1007/BF01731376. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Trentham D. R. The twelfth Colworth Medal lecture. The adenosine triphosphatase reactions of myosin and actomyosin and their relation to energy transduction in muscle. Biochem Soc Trans. 1977;5(1):5–22. doi: 10.1042/bst0050005. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]