Abstract
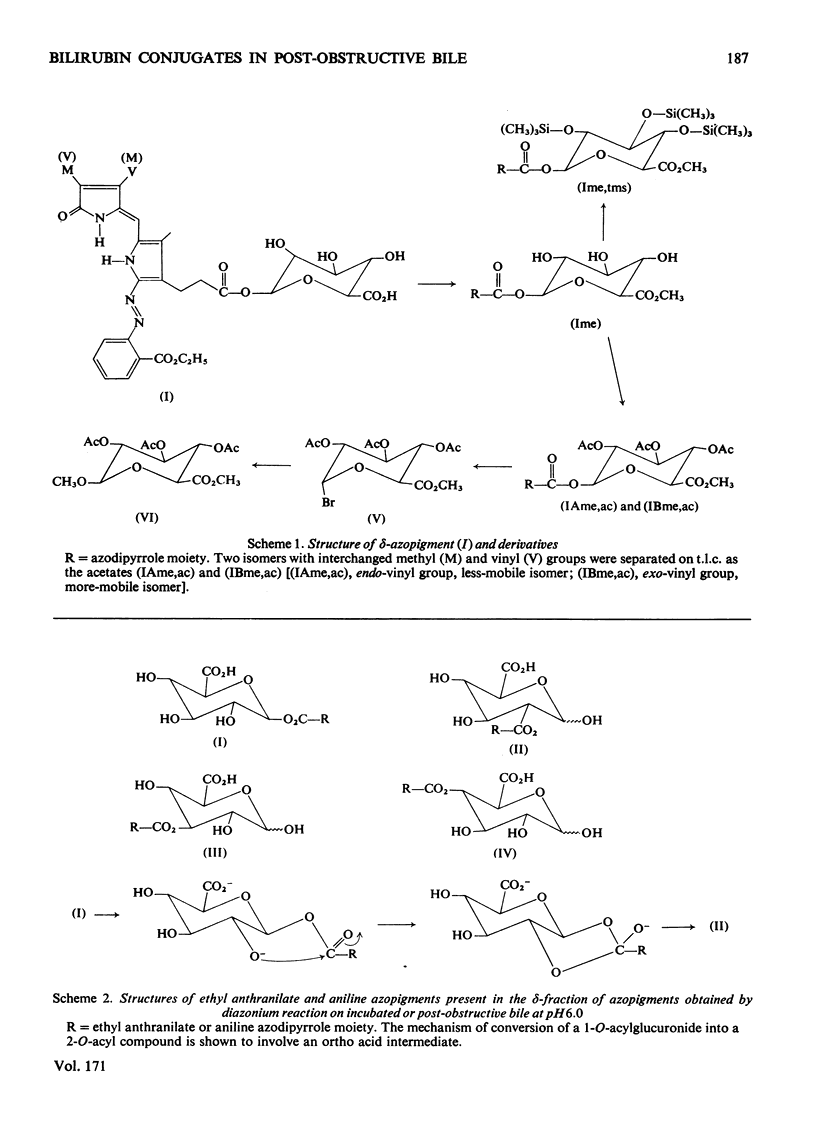
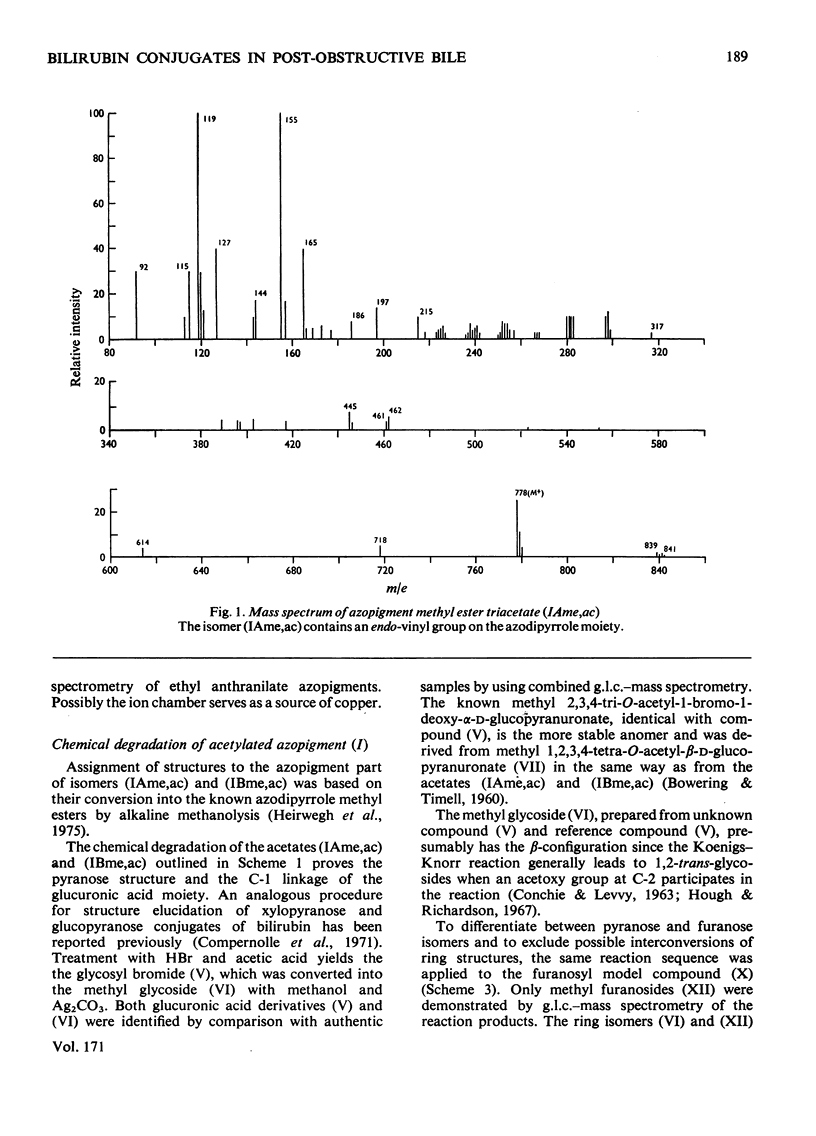
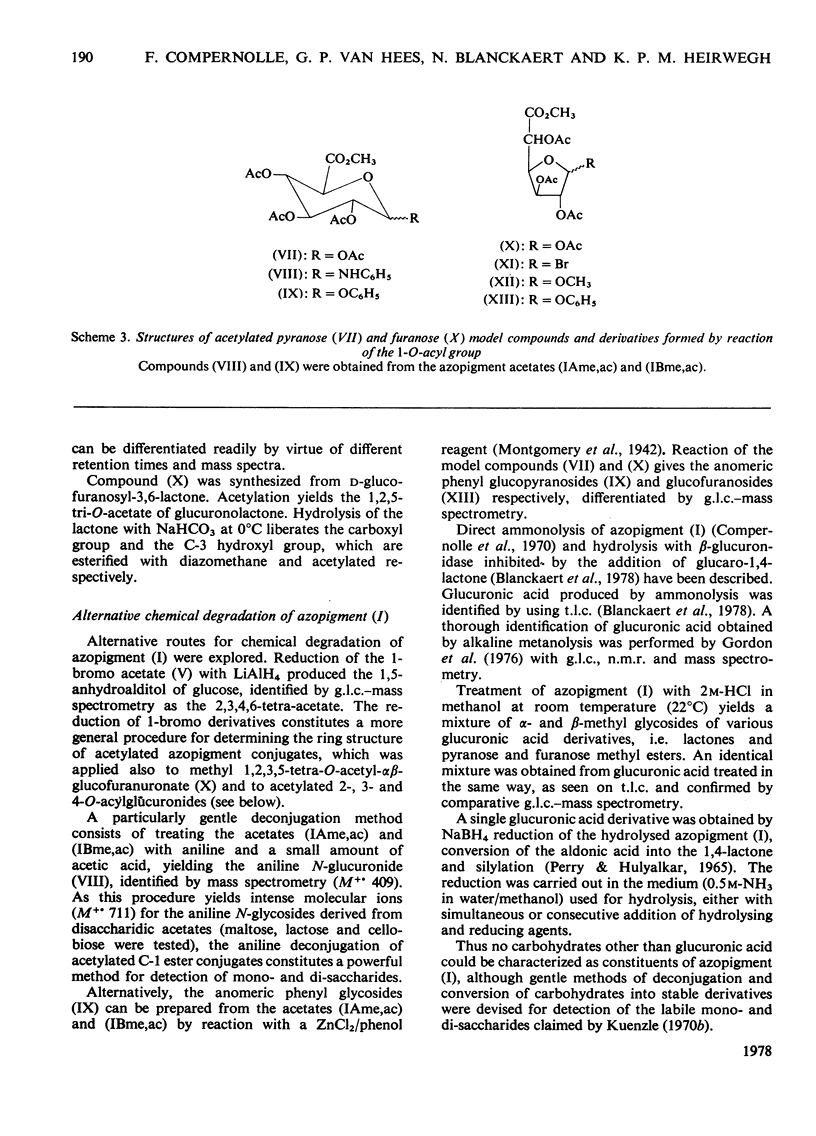
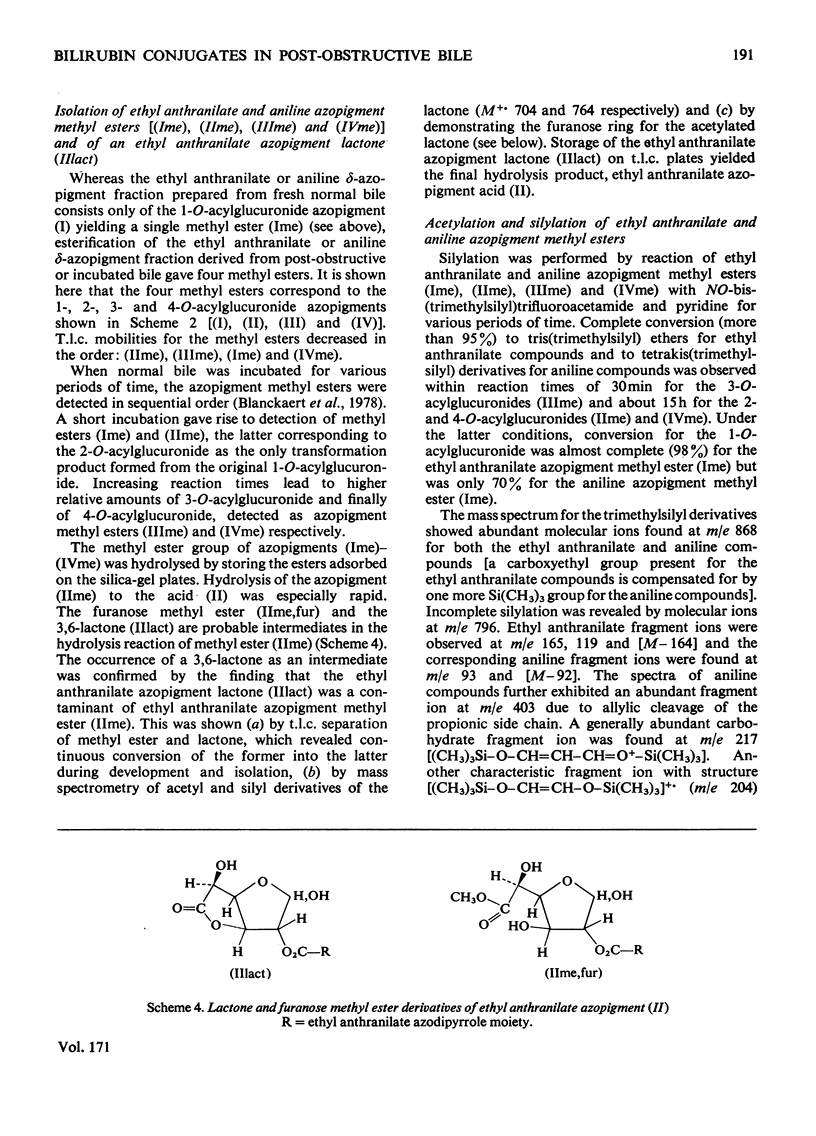
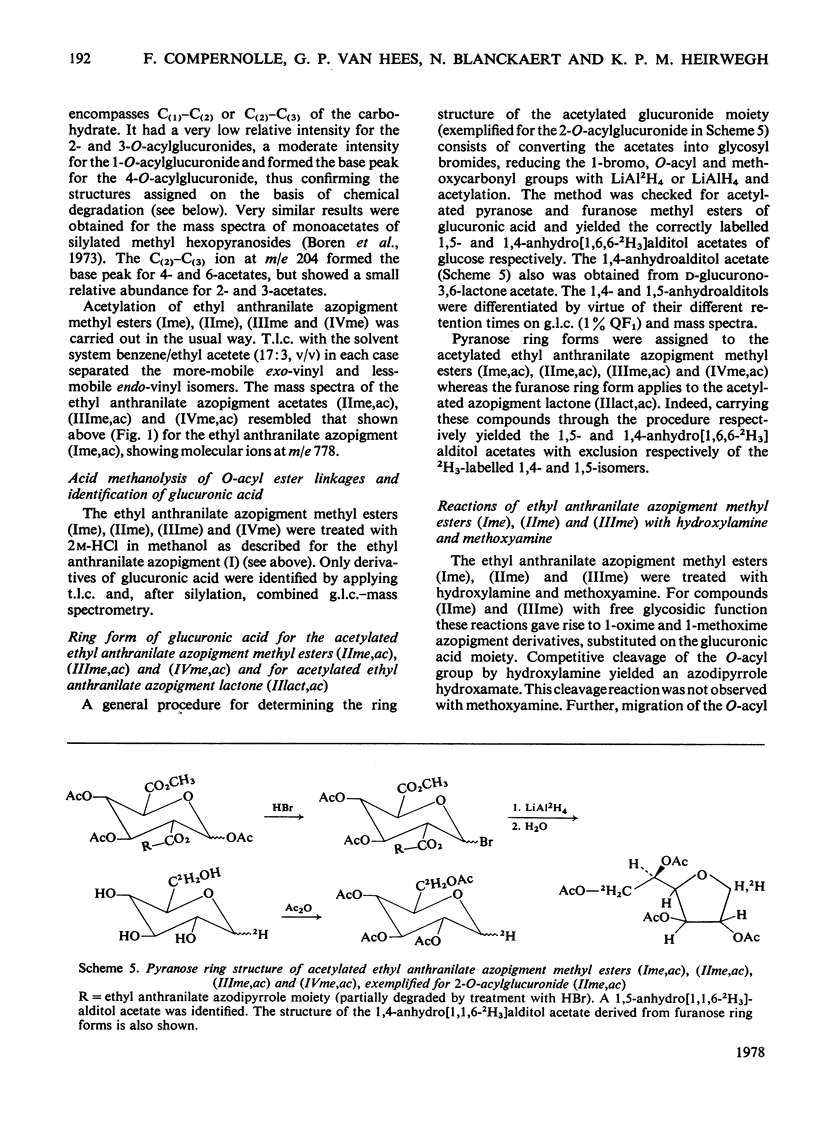
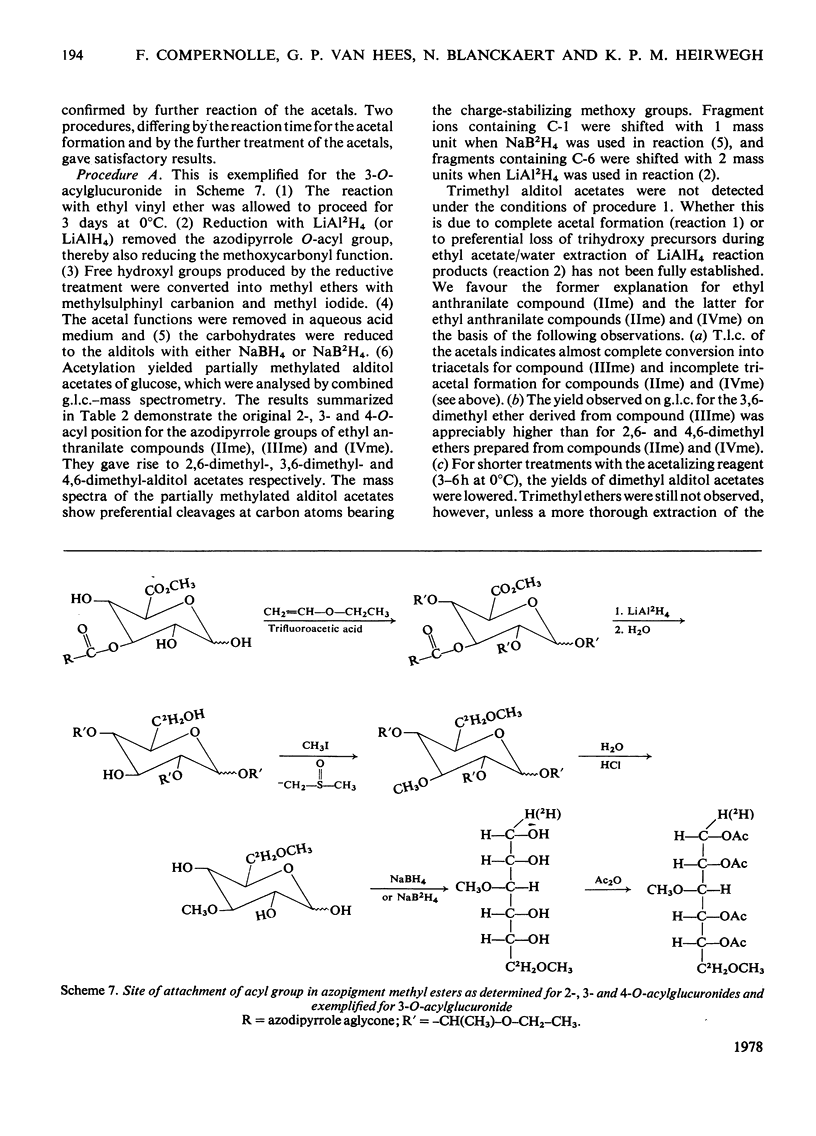
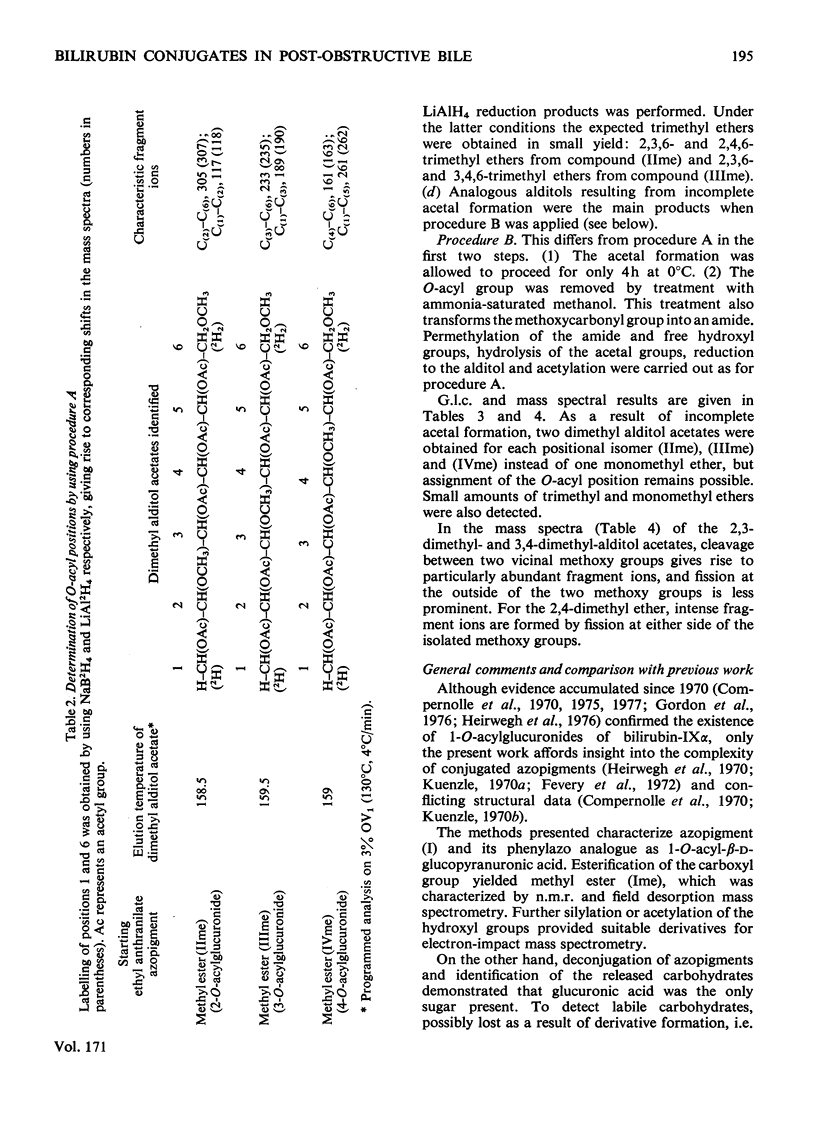
Structures have been determined for bilirubin-IXα conjugates in freshly collected bile of normal rats, dogs and man and in post-obstructive bile of man and rats. The originally secreted conjugate has been characterized as azopigment (I), i.e. a 1-O-acyl-β-d-glucopyranuronic acid glycoside. Conversion of the acetylated methyl ester of azopigment (I) into methyl 2,3,4-tri-O-acetyl-1-bromo-1-deoxy-β-d-glucopyranuronate (V) indicates the pyranose ring structure for the carbohydrate and a C-1 attachment for the bilirubin-IXα acyl group. Alternative procedures for deconjugation of azopigment (I) and its derivatives are also described. In post-obstructive bile, the 1-O-acylglucuronide is converted into 2-, 3- and 4-O-acylglucuronides via sequential intramolecular migrations of the bilirubin acyl group. The following approach was utilized. (1) The tetrapyrrole conjugates were cleaved to dipyrrolic aniline and ethyl anthranilate azopigments, and the azopigments were separated as the acids or methyl esters. (2) The isomeric methyl esters were characterized by mass spectral analysis of the acetates and silyl ethers. (3) The free glycosidic function was demonstrated by 1-oxime and 1-methoxime derivative formation. (4) The position of the dipyrrolic O-acyl group was determined for the methyl esters by protecting the free hydroxyl groups of the glucuronic acid moieties as the acetals formed with ethyl vinyl ether and by further conversion of the carbohydrates into partially methylated alditol acetates. These were analysed by using g.l.c.–mass spectrometry. The relevance of the present results with regard to previous reports on disaccharidic conjugates is discussed. Details of procedures for the formation of chemical derivatives for g.l.c. and mass spectrometry have been deposited as Supplementary Publication SUP 50081 (15 pages) at the British Library Lending Division, Boston Spa, West Yorkshire LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1978), 169, 5.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLING B. H., COLE P. G., LATHE G. H. The excretion of bilirubin as a diglucuronide giving the direct van den Bergh reaction. Biochem J. 1957 Apr;65(4):774–784. doi: 10.1042/bj0650774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Compernolle F., Leroy P., Van Houtte R., Fevery J., Heirwegh K. P. The fate of bilirubin-IXalpha glucuronide in cholestasis and during storage in vitro. Intramolecular rearrangement to positional isomers of glucuronic acid. Biochem J. 1978 Apr 1;171(1):203–214. doi: 10.1042/bj1710203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanckaert N., Fevery J., Heirwegh K. P., Compernolle F. Characterization of the major diazo-positive pigments in bile of homozygous Gunn rats. Biochem J. 1977 Apr 15;164(1):237–249. doi: 10.1042/bj1640237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Blanckaert N., Heirwegh K. P. Mass spectral study of derivatives of the four bilirubin-IX isomers. Biomed Mass Spectrom. 1976 Aug;3(4):155–160. doi: 10.1002/bms.1200030403. [DOI] [PubMed] [Google Scholar]
- Compernolle F., Blanckaert N., Heirwegh K. P. The fate of bilirubin-IXalpha glucuronides in cholestatic bile: sequential migration of the 1-acylaglycone to the 2-, 3- and 4- positions of glucuronic acid. Biochem Soc Trans. 1977;5(1):317–319. doi: 10.1042/bst0050317. [DOI] [PubMed] [Google Scholar]
- Compernolle F., Jansen F. H., Heirwegh K. P. Mass-spectrometric study of the azopigments obtained from bile pigments with diazotized ethyl anthranilate. Biochem J. 1970 Dec;120(4):891–894. doi: 10.1042/bj1200891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Fevery J., Heirwegh K. P. Mass-spectrometric structure elucidation of dog bile azopigments as the acyl glycosides of glucopyranose and xylopyranose. Biochem J. 1971 Dec;125(3):811–819. doi: 10.1042/bj1250811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fevery J., Van Damme B., Michiels R., De Groote J., Heirwegh K. P. Bilirubin conjugates in bile of man and rat in the normal state and in liver disease. J Clin Invest. 1972 Sep;51(9):2482–2492. doi: 10.1172/JCI107062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R., Chan T. H., Samodai K., Goresky C. A. The isolation and further characterization of the bilirubin tetrapyrroles in bile-containing human duodenal juice and dog gall-bladder bile. Biochem J. 1977 Oct 1;167(1):1–8. doi: 10.1042/bj1670001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E. R., Goresky C. A., Chang T. H., Perlin A. S. The isolation and characterization of bilirubin diglucuronide, the major bilirubin conjugate in dog and human bile. Biochem J. 1976 Jun 1;155(3):477–486. doi: 10.1042/bj1550477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock F. E., Hutchinson D. W., Knell A. J. Synthetic conjugates of bilirubin. Biochem Soc Trans. 1976;4(2):303–304. doi: 10.1042/bst0040303. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Fevery J., Meuwissen J. A., De Groote J., Compernolle F., Desmet V., Van Roy F. P. Recent advances in the separation and analysis of diazo-positive bile pigments. Methods Biochem Anal. 1974;22:205–250. doi: 10.1002/9780470110423.ch5. [DOI] [PubMed] [Google Scholar]
- Heirwegh K. P., Fevery J., Michiels R., van Hees G. P., Compernolle F. Separation by thin-layer chromatography and structure elucidation of bilirubin conjugates isolated from dog bile. Biochem J. 1975 Feb;145(2):185–199. doi: 10.1042/bj1450185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heirwegh K. P., Van Hees G. P., Leroy P., Van Roy F. P., Jansen F. H. Heterogeneity of bile pigment conjugates as revealed by chromatography of their ethyl anthranilate azopigments. Biochem J. 1970 Dec;120(4):877–890. doi: 10.1042/bj1200877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. F., Soltmann B., Sweeley C. C. A model for ionization mechanisms in field desorption mass spectrometry. Biomed Mass Spectrom. 1976 Dec;3(6):340–345. doi: 10.1002/bms.1200030613. [DOI] [PubMed] [Google Scholar]
- Hutchinson D. W., Johnson B., Knell A. J. The synthesis of esters of bilirubin. Biochem J. 1973 Jul;133(3):493–498. doi: 10.1042/bj1330493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. Isolation of phenylazo derivatives of bile bilirubin. Biochem J. 1970 Sep;119(3):387–394. doi: 10.1042/bj1190387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzle C. C. Bilirubin conjugates of human bile. The excretion of bilirubin as the acyl glycosides of aldobiouronic acid, pseudoaldobiouronic acid and hexuronosylhexuronic acid, with a branched-chain hexuronic acid as one of the components of the hexuronosylhexuronide. Biochem J. 1970 Sep;119(3):411–435. doi: 10.1042/bj1190411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY M. B., HULYALKAR R. K. THE ANALYSIS OF HEXURONIC ACIDS IN BIOLOGICAL MATERIALS BY GAS-LIQUID PARTITION CHROMATOGRAPHY. Can J Biochem. 1965 May;43:573–584. doi: 10.1139/o65-067. [DOI] [PubMed] [Google Scholar]
- SCHMID R. The identification of direct-reacting bilirubin as bilirubin glucuronide. J Biol Chem. 1957 Dec;229(2):881–888. [PubMed] [Google Scholar]
- Salmon M., Fenselau C. Evaluation of bilirubin derivatives for mass spectral analysis. Biomed Mass Spectrom. 1974 Aug;1(4):219–222. doi: 10.1002/bms.1200010404. [DOI] [PubMed] [Google Scholar]
- TALAFANT E. Properties and composition of the bile pigment giving a direct diazo reaction. Nature. 1956 Aug 11;178(4528):312–312. doi: 10.1038/178312a0. [DOI] [PubMed] [Google Scholar]
- Thompson R. P., Hofmann A. F. Chemical synthesis of bilirubin glucuronide conjugates. Biochim Biophys Acta. 1976 Nov 18;451(1):267–277. doi: 10.1016/0304-4165(76)90277-4. [DOI] [PubMed] [Google Scholar]
- Van Roy F. P., Heirwegh K. P. Determination of bilirubin glucuronide and assay of glucuronyltransferase with bilirubin as acceptor. Biochem J. 1968 Apr;107(4):507–518. doi: 10.1042/bj1070507. [DOI] [PMC free article] [PubMed] [Google Scholar]


