Abstract
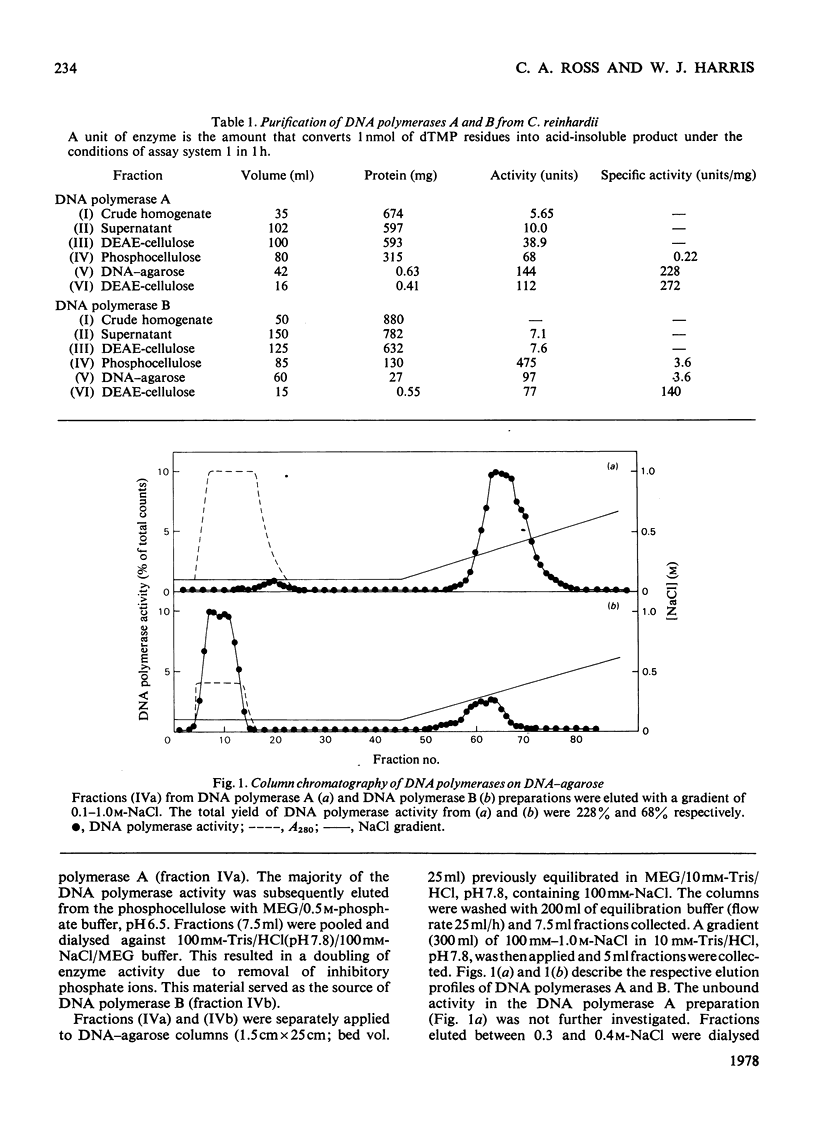
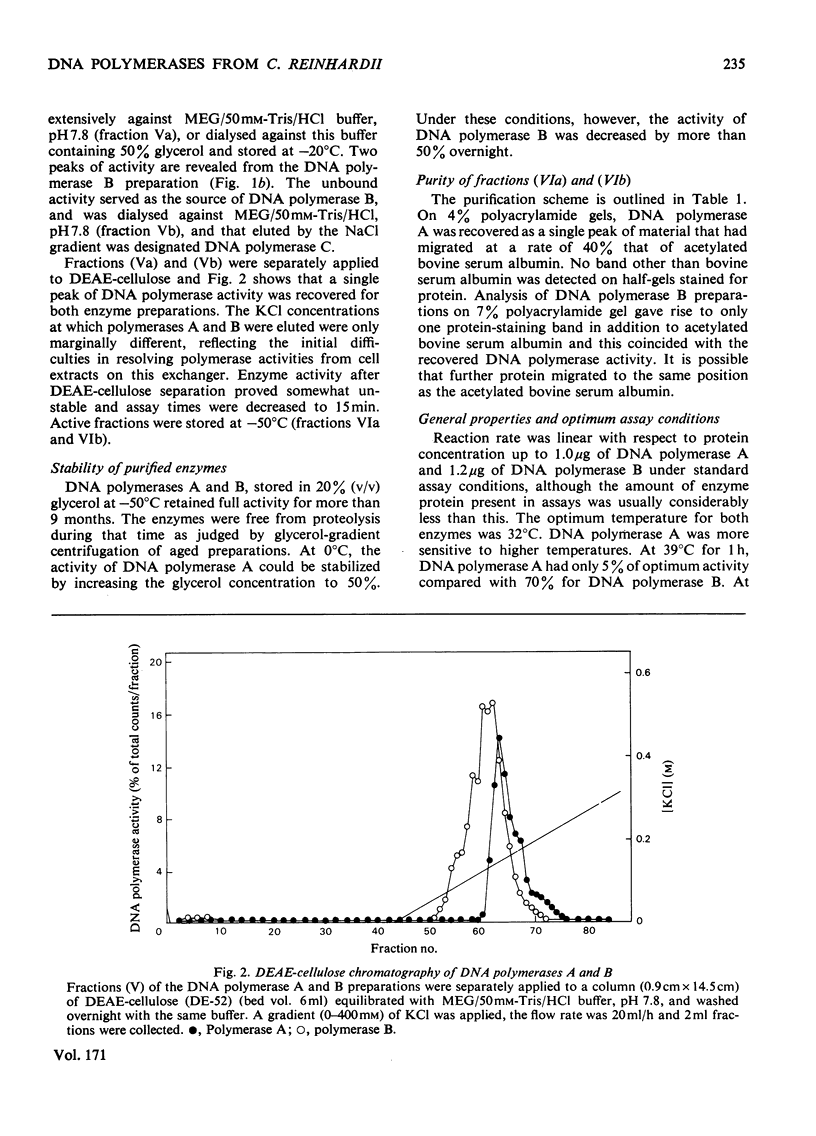
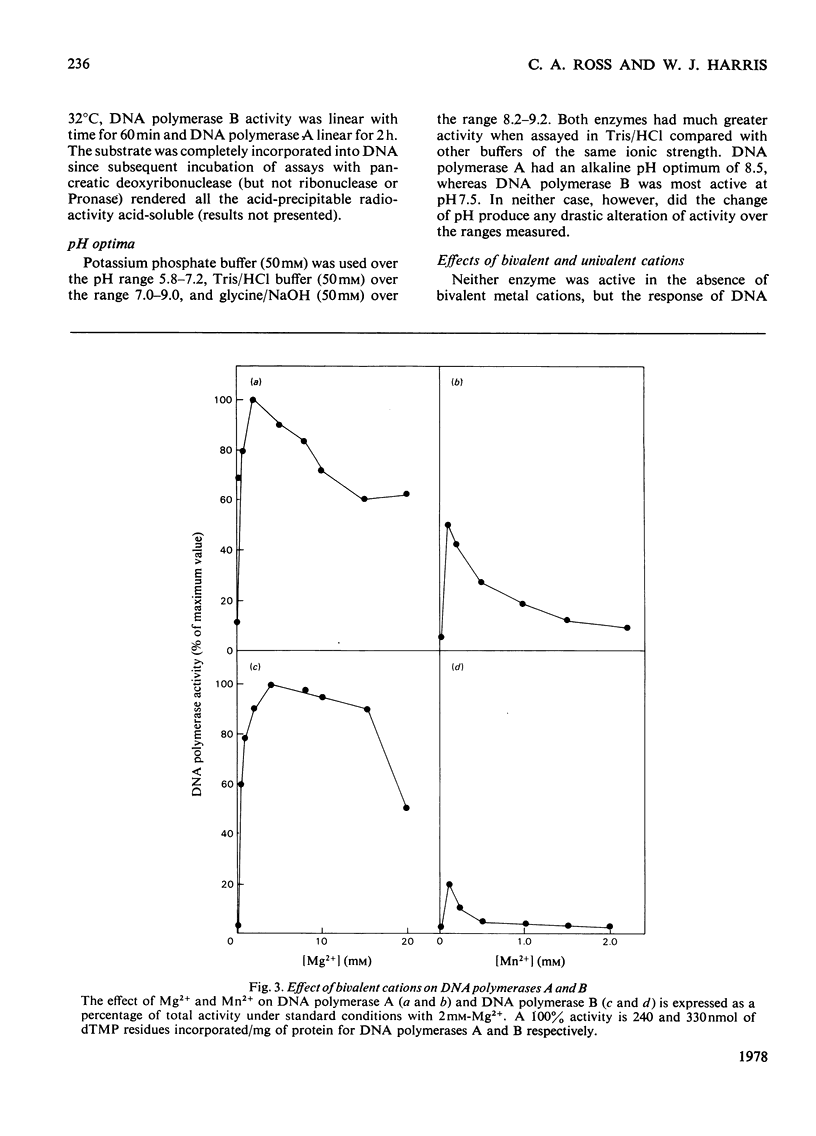
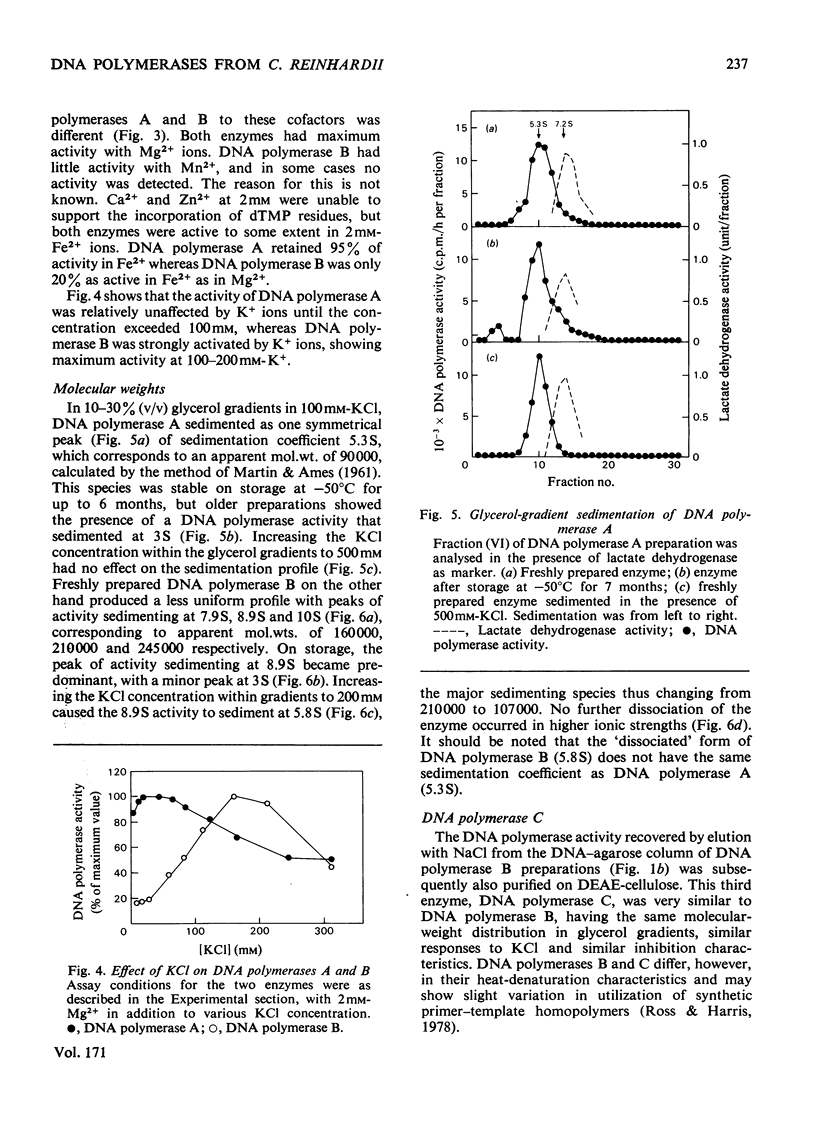
Three DNA polymerase activities, A, B and C, were identified in extracts of exponentially growing synchronous cultures of Chlamydomonas reinhardii, and DNA polymerases A and B were characterized in detail. Both enzymes have the same binding affinity for DEAE-cellulose at pH 7.8, but can be distinguished from each other by their behaviour on phosphocellulose and DNA-agarose. 'Activated' calf thymus DNA was used as template, and the pH, K+ and bivalent-cation optima were measured. DNA polymerase A sediments at 5.3 S in glycerol gradients, with an apparent mol.wt. of 90000-100000. Polymerase B sediments between 8S and 10S in 100mM-KCl, the predominant species having an apparent mol.wt. of 200000. In 200mM-KCl, polymerase B dissociates to a single species, which sediments at 5.8S. A 3S species was found in aged preparations of both enzymes. The activity of polymerase B from cells harvested during nuclear DNA synthesis is twice that found in Chlamydomonas at other times during the cell cycle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. R., Holloman W. K., Kairis M. V., Spanos A., Yarranton G. T. A DNA polymerase from Ustilago maydis. 1. Purification and properties of the polymerase activity. Eur J Biochem. 1976 Feb 2;62(1):131–142. doi: 10.1111/j.1432-1033.1976.tb10106.x. [DOI] [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., So A. G. Bone marrow cytoplasmic deoxyribonucleic acid polymerase. Variation of pH and ionic environment as a possible control mechanism. Biochemistry. 1973 Oct 23;12(22):4378–4384. doi: 10.1021/bi00746a013. [DOI] [PubMed] [Google Scholar]
- Chandra P., Steel L. K., Laube H., Kornhuber B. Oncornaviral-like DNA polymerase activity in a case of childhood myelofibrotic syndrome. FEBS Lett. 1975 Oct 15;58(1):71–75. doi: 10.1016/0014-5793(75)80228-6. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Brown M., Bollum F. J. Induction of DNA polymerase in mouse L cells. J Mol Biol. 1973 Feb 15;74(1):1–8. doi: 10.1016/0022-2836(73)90349-5. [DOI] [PubMed] [Google Scholar]
- Chang L. M. Phylogeny of DNA polymerase-beta. Science. 1976 Mar 19;191(4232):1183–1185. doi: 10.1126/science.769158. [DOI] [PubMed] [Google Scholar]
- Craig R. K., Costello P. A., Keir H. M. Dexyribonucleic acid polymerases of BHK-21/C13cells. Relationship to the physiological state of the cells, and to synchronous indution of synthesis of deoxyribonuleic acid. Biochem J. 1975 Feb;145(2):233–240. doi: 10.1042/bj1450233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crerar M., Pearlman R. E. Deoxyribonucleic acid polymerase from Tetrahymena pyriformis. Purification and properties of the major activity in exponentially growing cells. J Biol Chem. 1974 May 25;249(10):3123–3131. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gefter M. L. DNA replication. Annu Rev Biochem. 1975;44:45–78. doi: 10.1146/annurev.bi.44.070175.000401. [DOI] [PubMed] [Google Scholar]
- Gillham N. W. Genetic analysis of the chloroplast and mitochondrial genomes. Annu Rev Genet. 1974;8:347–391. doi: 10.1146/annurev.ge.08.120174.002023. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Hesslewood I. P., Johnston I. R. In vitro concersion of a calf thymus 8S DNA polymerase to a 7.3S species. Nature. 1975 May 29;255(5507):420–422. doi: 10.1038/255420a0. [DOI] [PubMed] [Google Scholar]
- Holmes A. M., Johnston I. R. DNA polymerases of eukaryotes. FEBS Lett. 1975 Dec 15;60(2):233–243. doi: 10.1016/0014-5793(75)80721-6. [DOI] [PubMed] [Google Scholar]
- Johnson U. G., Porter K. R. Fine structure of cell division in Chlamydomonas reinhardi. Basal bodies and microtubules. J Cell Biol. 1968 Aug;38(2):403–425. doi: 10.1083/jcb.38.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb L. A. Purification and properties of deoxyribonucleic acid polymerase from nuclei of sea urchin embryos. J Biol Chem. 1969 Apr 10;244(7):1672–1681. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Matsukage A., Sivarajan M., Wilson S. H. Studies on DNA alpha-polymerase of mouse myeloma: partial purification and comparison of three molecular forms of the enzyme. Biochemistry. 1976 Nov 30;15(24):5305–5314. doi: 10.1021/bi00669a017. [DOI] [PubMed] [Google Scholar]
- McLennan A. G., Keir H. M. Deoxyribonucleic acid polymerases of Euglena gracilis. Primer-template utilization of and enzyme activities associated with the two deoxyribonucleic acid polymerases of high molecular weight. Biochem J. 1975 Nov;151(2):239–247. doi: 10.1042/bj1510239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan A. G., Keir H. M. Deoxyribonucleic acid polymerases of Euglena gracilis. Purification and properties of two distinct deoxyribonucleic acid polymerases of high molecular weight. Biochem J. 1975 Nov;151(2):227–238. doi: 10.1042/bj1510227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan A. G., Keir H. M. Subcellular location and growth stage dependence of the DNA polymerases of Euglena gracilis. Biochim Biophys Acta. 1975 Oct 15;407(3):253–262. doi: 10.1016/0005-2787(75)90092-1. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Harris W. J. DNA polymerases from Chlamydomonas reinhardii. Further characterization, action of inhibitors and associated nuclease activities. Biochem J. 1978 Apr 1;171(1):241–249. doi: 10.1042/bj1710241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Harris W. J. Deoxyribonucleic acid polymerase from Chlamydomonas reinhardii. Biochem Soc Trans. 1976;4(4):806–807. doi: 10.1042/bst0040806. [DOI] [PubMed] [Google Scholar]
- Schaller H., Nüsslein C., Bonhoeffer F. J., Kurz C., Nietzschmann I. Affinity chromatography of DNA-binding enzymes on single-stranded DNA-agarose columns. Eur J Biochem. 1972 Apr 24;26(4):474–481. doi: 10.1111/j.1432-1033.1972.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Schönherr O. T., Keir H. M. The activity of deoxyribonucleic acid polymerase in some species of algae. Biochem J. 1972 Sep;129(2):285–290. doi: 10.1042/bj1290285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadari S., Weissbach A. HeLa cell R-deoxyribonucleic acid polymerases. Separation and characterization of two enzymatic activities. J Biol Chem. 1974 Sep 25;249(18):5809–5815. [PubMed] [Google Scholar]
- Stavrianopoulos J. G., Karkas J. D., Chargaff E. DNA polymerase of chicken embryo: purification and properties. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1781–1785. doi: 10.1073/pnas.69.7.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Chiang K. S., Kates J. R. Deoxyribonucleic acid replication in meiosis of Chlamydomonas reinhardi. I. Isotopic transfer experiments with a strain producing eight zoospores. J Mol Biol. 1967 Apr 14;25(1):47–66. doi: 10.1016/0022-2836(67)90278-1. [DOI] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait A., Cummings D. J. DNA-dependent DNA polymerase activities from Paramecia macronuclei. Biochim Biophys Acta. 1975 Jan 20;378(2):282–295. doi: 10.1016/0005-2787(75)90116-1. [DOI] [PubMed] [Google Scholar]
- Tait G. C., Harris W. J. A deoxyribonuclease from Chlamydomonas reinhardii. 1. Purification and properties. Eur J Biochem. 1977 May 16;75(2):357–364. doi: 10.1111/j.1432-1033.1977.tb11536.x. [DOI] [PubMed] [Google Scholar]
- Weissbach A., Baltimore D., Bollum F., Gallo R., Korn D. Nomenclature of eukaryotic DNA polymerases. Eur J Biochem. 1975 Nov 1;59(1):1–2. doi: 10.1111/j.1432-1033.1975.tb02416.x. [DOI] [PubMed] [Google Scholar]
- Weissbach A. Vertebrate DNA polymerases. Cell. 1975 Jun;5(2):101–108. doi: 10.1016/0092-8674(75)90017-3. [DOI] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintersberger E. Deoxyribonucleic acid polymerases from yeast. Further purification and characterization of DNA-dependent DNA polymerases A and B. Eur J Biochem. 1974 Dec 16;50(1):41–47. doi: 10.1111/j.1432-1033.1974.tb03871.x. [DOI] [PubMed] [Google Scholar]
- Wintersberger U. Absence of a low-molecular-weight DNA polymerase from nuclei of the yeast, Saccharomyces cerevisiae. Eur J Biochem. 1974 Dec 16;50(1):197–202. doi: 10.1111/j.1432-1033.1974.tb03888.x. [DOI] [PubMed] [Google Scholar]
- Wintersberger U., Wintersberger E. Studies on deoxyribonucleic acid polymerases from yeast. 2. Partial purification and characterization of mitochondrial DNA polymerase from wild type and respiration-deficient yeast cells. Eur J Biochem. 1970 Mar 1;13(1):20–27. doi: 10.1111/j.1432-1033.1970.tb00894.x. [DOI] [PubMed] [Google Scholar]



