Abstract
1. Myofibrils from human skeletal muscle contained regulatory proteins exhibiting similar electrophoretic behaviour to those present in rabbit skeletal muscle. 2. All human skeletal muscles examined contained two forms of troponin I corresponding to the forms already characterized in fast and slow rabbit muscle. 3. The ratios of the amounts of the two forms of troponin I in different human skeletal muscles were not identical with the ratios for the type 1 to type 2 fibres published in the literature. The ratios could, however, be arranged in the same rank order. 4. Primate heart contained a single form of troponin I different in molecular weight and amino acid composition from the skeletal forms. 5. A monospecific antiserum to human cardiac troponin I was prepared in the sheep and shown not to react with the fast or slow skeletal-muscle forms of troponin I from human and other species. 6. The anti-(human cardiac-muscle troponin I) reacted with the cardiac troponin I from the human, baboon, rabbit and rhesus monkey. Positive reactions were also obtained with urea extracts of whole cardiac tissue.
Full text
PDF



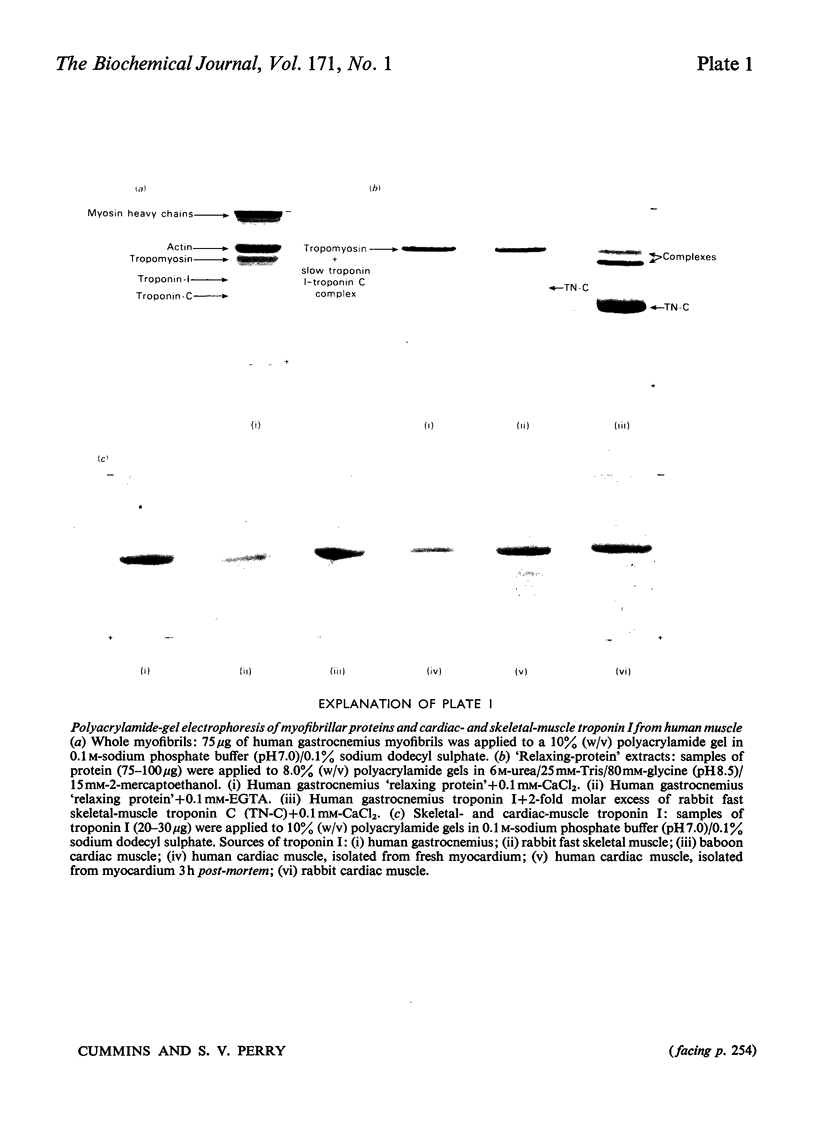

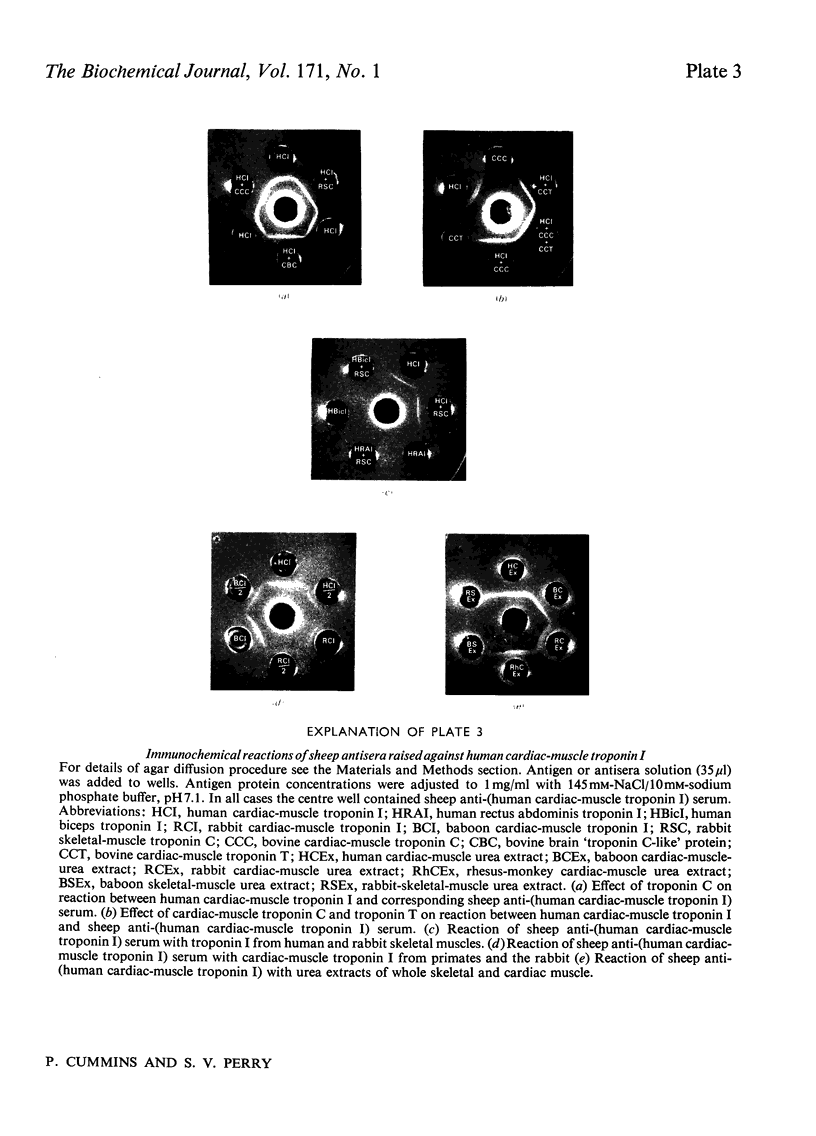




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amphlett G. W., Perry S. V., Syska H., Brown M. D., Vrbova G. Cross innervation and the regulatory protein system of rabbit soleus muscle. Nature. 1975 Oct 16;257(5527):602–604. doi: 10.1038/257602a0. [DOI] [PubMed] [Google Scholar]
- Clarke F. M., Lovell S. J., Masters C. J., Winzor D. J. Beef muscle troponin: evidence for multiple forms of troponin-T. Biochim Biophys Acta. 1976 Apr 14;427(2):617–626. doi: 10.1016/0005-2795(76)90205-1. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin light chains, troponin-C and parvalbumins deduced from comparison of their amino acid sequences. Biochem Biophys Res Commun. 1974 May 7;58(1):301–308. doi: 10.1016/0006-291x(74)90927-9. [DOI] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. Chemical and immunochemical characteristics of tropomyosins from striated and smooth muscle. Biochem J. 1974 Jul;141(1):43–49. doi: 10.1042/bj1410043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Perry S. V. The subunits and biological activity of polymorphic forms of tropomyosin. Biochem J. 1973 Aug;133(4):765–777. doi: 10.1042/bj1330765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Wakabayashi T., Ebashi F. Troponin and its components. J Biochem. 1971 Feb;69(2):441–445. doi: 10.1093/oxfordjournals.jbchem.a129486. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Maron B. J., Adelstein R. S. Human heart and platelet actins are products of different genes. Science. 1976 Jan 9;191(4222):94–95. doi: 10.1126/science.1246600. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Wilkinson J. M. The amino acid sequence of rabbit cardiac troponin I. Biochem J. 1976 Dec 1;159(3):633–641. doi: 10.1042/bj1590633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F., Perry S. V. The interaction of the calcium-binding protein (troponin C) with bivalent cations and the inhibitory protein (troponin I). Biochem J. 1974 Feb;137(2):145–154. doi: 10.1042/bj1370145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F., Weeks R. A., Perry S. V. Affinity-chromatographic isolation and some properties of troponin C from different muscle types. Biochem J. 1977 Mar 1;161(3):465–471. doi: 10.1042/bj1610465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi T., Perry S. V. An immunochemical study of the calcium ion-binding protein (troponin-C) and the inhibitory protein (troponin-I) of the troponin complex and their interaction. Biochim Biophys Acta. 1974 Jun 7;351(2):273–289. doi: 10.1016/0005-2795(74)90189-5. [DOI] [PubMed] [Google Scholar]
- Hitchcock S. E. Regulation of muscle contraction. Effect of calcium on the affinity of troponin for actin and tropomyosin. Biochemistry. 1973 Jun 19;12(13):2509–2513. doi: 10.1021/bi00737a022. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Cohen C. Letter: Troponin subunit interactions. J Mol Biol. 1973 Dec 15;81(3):409–413. doi: 10.1016/0022-2836(73)90150-2. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Pearlstone J. R., Carpenter M. R., Johnson P., Smillie L. B. Amino-acid sequence of tropomyosin-binding component of rabbit skeletal muscle troponin. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1902–1906. doi: 10.1073/pnas.73.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Cole H. A. Phosphorylation of troponin and the effects of interactions between the components of the complex. Biochem J. 1974 Sep;141(3):733–743. doi: 10.1042/bj1410733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Perry S. V. The relaxing protein system of striated muscle. Resolution of the troponin complex into inhibitory and calcium ion-sensitizing factors and their relationship to tropomyosin. Biochem J. 1969 Dec;115(5):993–1004. doi: 10.1042/bj1150993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Syska H., Perry S. V., Trayer I. P. A new method of preparation of troponin I (inhibitory protein) using affinity chromatography. Evidence for three different forms of troponin I in striated muscle. FEBS Lett. 1974 Apr 1;40(2):253–257. doi: 10.1016/0014-5793(74)80238-3. [DOI] [PubMed] [Google Scholar]
- Syska H., Wilkinson J. M., Grand R. J., Perry S. V. The relationship between biological activity and primary structure of troponin I from white skeletal muscle of the rabbit. Biochem J. 1976 Feb 1;153(2):375–387. doi: 10.1042/bj1530375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. W., Essén B., Saltin B. Myosin ATPase in skeletal muscle of healthy men. Acta Physiol Scand. 1974 Aug;91(4):568–570. doi: 10.1111/j.1748-1716.1974.tb05712.x. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wilkinson J. M., Grand R. J. The amino acid sequence of troponin I from rabbit skeletal muscle. Biochem J. 1975 Aug;149(2):493–496. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M. Isolation and purification of the cyanogen bromide fragments from troponin I. FEBS Lett. 1974 Apr 15;41(1):166–168. doi: 10.1016/0014-5793(74)80979-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Perry S. V., Cole H. A., Trayer I. P. The regulatory proteins of the myofibril. Separation and biological activity of the components of inhibitory-factor preparations. Biochem J. 1972 Mar;127(1):215–228. doi: 10.1042/bj1270215. [DOI] [PMC free article] [PubMed] [Google Scholar]





