Abstract
Background
Approaches for determining whether influenza vaccination prevents infection, attenuates illness, or both are important for developing improved vaccines. We estimated influenza infection incidence and evaluated symptom ascertainment methodologies in children to inform future vaccine trial design.
Methods
We conducted a prospective cohort study among children aged 6 to 23 months from May to October 2022. Study nurses collected symptom and temperature data and midturbinate nasal swabs twice weekly irrespective of symptoms; caregivers entered symptom data daily and collected nasal swabs weekly. Samples were tested for influenza with polymerase chain reaction.
Results
Of 230 healthy screened children, 93 were enrolled, of whom 87 (94%) completed 6-month follow-up. In total, 95% (4245/4476) of scheduled nurses, 90% (2045/2276) of caregiver swabs, 99% (92/93) of baseline blood collections, and 67% (9245/13 768) of scheduled symptom diaries were completed. Polymerase chain reaction–confirmed influenza incidence was 65% (60/93) for ≥1 infection; 11 (18%) individuals had 2 episodes and 1 (2%) had 3. Of 73 episodes, 55 (75%) had ≥1 symptom and 37 (51%) had fever (measured and/or reported). Median infection duration was 7 days (IQR, 4–9). Human RNase P gene was detected in 99% (2032/2045) of caregiver-collected swabs, through which 5 additional episodes were identified. Per episode, caregivers' diaries of reported and measured fever were 19% (25/73, 34%) and 11% (15/73, 21%) higher than nurse-reported (11/73, 15%) and nurse-measured (7/73, 10%) fever, respectively.
Conclusions
The incidence of influenza infection was high and mainly symptomatic, suggesting that this platform could be suitable for future trials of vaccine efficacy and correlates of protection against infection and illness in children.
Keywords: influenza, South Africa, symptoms, transmission, vaccine
In a cohort of 6- to 23-month-old children in South Africa who were swabbed twice weekly irrespective of symptoms, influenza infection incidence was high and mainly symptomatic, suggesting that this platform could be suitable for future trials of vaccine efficacy and correlates of protection.
Despite the wide global usage of influenza vaccines, it remains unknown whether the mechanism of illness prevention is through prevention of infection, attenuation of illness given infection, or both [1]. A recent review article concluded that influenza vaccination may attenuate disease severity in breakthrough infections, but more studies are needed to examine this question. If influenza vaccines prevent infection and/or are associated with reduced intensity and duration of shedding with breakthrough infections, this could translate into reductions in transmission. Platforms for the ascertainment of influenza infection and illness incidence will be critical for understanding the mechanism of vaccine protection and to identify correlates of protection. This will be important for guiding target groups and developing improved influenza vaccines.
In previous studies, we have optimized a novel approach to identify the burden of symptomatic and asymptomatic influenza infections through twice-weekly collection and testing of upper respiratory swabs by reverse transcription real-time polymerase chain reaction (rRT-PCR) irrespective of symptoms [2, 3]. This could provide a suitable platform for the evaluation of the efficacy of new vaccines and correlates of protection against infection and illness. Caregiver collection of swabs and symptom ascertainment could potentially reduce costs.
In South Africa, the highest rates of influenza are among young children [4], who are also important drivers of household transmission. Currently, children aged 6 to 23 months are not a priority group for publicly funded vaccination, although influenza vaccination is recommended for this group if privately funded [5].
We aimed to explore different approaches to measuring influenza incidence and symptoms in children aged 6 to 23 months in an urban South African community, with the intention to develop more standardized and simpler approaches for measurement of infection and illness outcomes. This may facilitate future clinical trials aiming to evaluate whether vaccination against influenza prevents infection or attenuates illness severity.
METHODS
Study Design and Setting
We conducted a prospective cohort study (the Identifying Symptoms in Toddlers study) in Jouberton, a township outside Klerksdorp in the North West province of South Africa, the site of several previous studies of community influenza and SARS-CoV-2 transmission [3, 4, 6]. Inactivated influenza vaccine coverage among children aged <5 years is <0.1%, a reflection of the national coverage [6].
Participants
We enrolled healthy resident children of Jouberton township aged 6 to 23 months who were HIV uninfected and available for 6 months of follow-up. We aimed to enroll 100 children to achieve a goal of 50 evaluable children. This sample size was pragmatically chosen given available resources.
Enrollment and Follow-up
We identified potentially eligible children by prescreening attendees at child vaccination and well-baby clinics or those children accompanying ill mothers or siblings during clinic visits from January through March 2022. Following parental informed consent, we collected baseline data, including demographic data, and screened for underlying illness using questionnaires and clinical examination, including HIV infection, which is routinely established in infancy for all children born in South Africa. At enrollment, we trained caregivers on study procedures such as collection and packaging of midturbinate nasal swab samples, daily temperature, heart rate and oxygen saturation measurement, and daily electronic symptom questionnaire completion. Refresher training was provided if irregular measurements were submitted during follow-up.
Follow-up was from 2 May through 30 October 2022 and involved the following components: (1) twice-weekly nurse household visits (Monday and Thursday or Tuesday and Friday) with nurse collection of midturbinate nasal swabs, measurement of tympanic temperature, heart rate and oxygen saturation measurement, and administration of a structured questionnaire to assess the presence of symptoms and health care–seeking behavior and (2) once-weekly parent/caregiver collection of a midturbinate nasal swab from the child, placed in universal transport medium (Saturday or Sunday depending on visit schedule). The swab was packaged with ice packs in a cooler box and collected on the same day by a motorcycle courier, who transported the sample on ice to the study laboratory. Once daily, the parent/caregiver completed an electronic symptom questionnaire on a study-provided tablet and recorded the child's tympanic temperature, heart rate, and oxygen saturation. Nurses collected blood at the start and end of follow-up. Participants needing medical attention were referred to their local clinics for further care at any time during the study.
Laboratory Procedures
All samples were refrigerated (2–8 °C) and transported within 24 hours to the analytical laboratory at the National Institute for Communicable Diseases, South Africa. Total nucleic acids were extracted with the Roche MagNA Pure 96 instrument (Roche Diagnostics) and MP96 DNA and Viral NA SV Kit (Roche Diagnostics). Extracts were tested for influenza A and B viruses by rRT-PCR with the Allplex SARS-CoV-2/FluA/FluB/RSV multiplex commercial kit (Seegene). A specimen was considered positive for SARS-CoV-2/FluA/FluB/RSV if the targets were detected with cycle threshold (Ct) values ≤40 according to manufacturer instructions. Samples positive for influenza A and B were subtyped by the CDC influenza A (H1/H3/H1pdm09) subtyping and the CDC B/Yamagata-B/Victoria lineage typing kits, respectively.
Blood samples were tested for rise in influenza antibody titers with hemagglutination inhibition (HAI) assays against reference influenza virus antigens based on the selected vaccine strains and strains predominantly circulating in South Africa during 2022 [7].
Data Management
All data, including laboratory results, were collected electronically in real time via REDCap hosted at the University of the Witwatersrand [8, 9].
Analysis
An episode of influenza infection (shedding) was defined as ≥1 rRT-PCR positive nasal swab for influenza. The duration of shedding was the difference in days between the first and last days of rRT-PCR positivity. If an individual tested positive for a different influenza subtype or lineage or the same subtype or lineage ≥2 weeks after the last day of a prior episode, it was considered a separate infection episode. An illness episode was defined as an episode with ≥1 nurse- or caregiver-reported symptoms in the period from 1 nurse visit before to 1 visit after the influenza infection episode. Incidence of rRT-PCR–confirmed infection and symptomatic illness with influenza was computed as the number of infection and illness episodes divided by the person-seasons of follow-up (considering the 6-month follow-up period as 1 season). The symptomatic fraction was computed as the number of infections reporting ≥1 symptom divided by the total number of infection episodes. We assessed factors associated with influenza infection and symptomatic illness using logistic regression and factors associated with shedding duration and generation interval using Weibull accelerated time failure regression. Proportions were compared by Fisher exact test.
Seroconversion for influenza was considered at least a 4-fold rise in HAI titers between baseline and study end. Seroconversion rates were compared with rRT-PCR–confirmed infection by influenza subtype or lineage.
Completeness of symptom diaries was computed as the proportion of daily entries completed over the total expected entries. We compared (1) the proportion of influenza episodes with symptoms reported in daily diaries during the period from the first through last PCR-positive test results of the episode and (2) the proportion of influenza episodes with symptoms reported in twice-weekly nurse reports. Missing symptom reports were analyzed as absent symptoms.
Sensitivity of twice-weekly nurse swabs vs thrice-weekly swabs, including caregiver-administered weekend swab for detection of infection with influenza, was assessed by comparing the influenza incidence of a first episode of infection (percentage of participants with ≥1 influenza episode) in each of the 2 groups with the incidence when considering both groups via computation of the κ statistic.
RESULTS
Enrollment
Of 230 children screened for study eligibility, 100 were enrolled as participants. Reasons for exclusion included no parent or legal guardian available to sign informed consent (n = 43), ill children (n = 42), and age ineligibility (n = 31; Supplementary Figure 1). Of the 100 enrolled individuals, 7 did not complete their first follow-up visit. Of the 93 who did, 87 (94%) were followed to the final study visit. Reasons for not completing follow-up included relocation (n = 5) and voluntary withdrawal (n = 1).
Participant Characteristics
Of the 93 children included in the study, their median age was 12 months (IQR, 9–14) and 49 (53%) were male (Table 1, Supplementary Table 1). The median caregiver age was 30 years (range, 24–35), and the majority (n = 81, 87%) were unemployed and had some secondary education (n = 56, 60%). The median household size was 5 individuals (range, 4–6), and 62 households (67%) reported crowding (>2 people per sleeping room).
Table 1.
Individual, Household, and Caregiver Characteristics of Children and Factors Associated With Influenza Infection: Identifying Symptoms in Toddlers Study, South Africa, 2022
| No. (%) | Analysis | ||||
|---|---|---|---|---|---|
| Characteristic | Overall (n = 93) | Influenza Uninfected (n = 33) | Influenza Infected (n = 60) | Univariate, OR (95% CI) | Multivariable, aOR (95% CI) |
| Age, mo | |||||
| 6–11 | 44 (47) | 14 (42) | 30 (50) | 1.4 (.4–4.7) | … |
| 12–17 | 34 (37) | 13 (39) | 21 (35) | 1.1 (.3–3.7) | … |
| 18–23 | 15 (16) | 6 (18) | 9 (15) | 1 [Reference] | … |
| Sex | |||||
| Female | 44 (47) | 20 (61) | 24 (40) | 1 [Reference] | 1 [Reference] |
| Male | 49 (53) | 13 (39) | 36 (60) | 2.3 (1.0–5.6) | 2.6 (1.1–6.7) |
| Childcare attendance | |||||
| Yes | 7 (8) | 1 (3) | 6 (10) | 1 [Reference] | … |
| No | 86 (92) | 32 (97) | 54 (90) | 3.6 (.6–6.8) | … |
| Mother's HIV status | |||||
| Positive | 26 (28) | 5 (15) | 21 (35) | 3.0 (1.1–9.9) | 3.5 (1.2–11.8) |
| Negative | 67 (72) | 28 (85) | 39 (65) | 1 [Reference] | 1 [Reference] |
| Caregiver highest education level | |||||
| No schooling | 2 (2) | 2 (6) | 0 (0) | Not estimated | … |
| Primary | 3 (3) | 1 (3) | 2 (3) | 1.7 (.1–4.0) | … |
| Some secondary | 56 (60) | 17 (52) | 39 (65) | 2.0 (.8–5.2) | … |
| Completed secondary | 26 (28) | 12 (36) | 14 (23) | 1 [Reference] | … |
| Postsecondary | 6 (7) | 1 (3) | 5 (8) | 4.3 (.6–8.8) | … |
| Household size | |||||
| 2–5 | 54 (58) | 19 (58) | 35 (58) | 1 [Reference] | … |
| 6–9 | 36 (39) | 12 (36) | 24 (40) | 3.7 (.3–8.2) | … |
| 10–13 | 3 (3) | 2 (6) | 1 (2) | 4.0 (.3–9.1) | … |
| Crowdinga | |||||
| Yes | 62 (67) | 23 (70) | 39 (65) | 1 [Reference] | … |
| No | 31 (33) | 10 (30) | 21 (35) | 1.2 (.5–3.2) | … |
a>2 people per sleeping room.
Follow-up
Of 4369 scheduled nurse swabs during follow-up, a swab was collected for 4245 (97%), and of 2203 scheduled caregiver swabs, 2045 (93%) were collected (P = .23; overall completeness, 6290/6572, 96%; Supplementary Table 2). The human RNase P gene was detected in >99% (4239/4245) and 99% (2032/2045) of nurse- and caregiver-collected swabs, respectively. Influenza was identified on rRT-PCR from 4% (262/6028) of collected swabs.
Most scheduled nurse visits (97%, 4247/4369) were completed (Supplementary Table 2). Of these, 100% (n = 4247) had symptom data recorded, 96% (n = 4070) body temperature, 80% (n = 3414) blood oxygen saturation, and 80% (n = 3414) heart rate. Symptom diaries were completed by caregivers for only 67% (9245/13 768) of scheduled instances. Of these instances, 100% (n = 9244) had symptom and body temperature data, and 82% (n = 7587) had blood oxygen saturation and 82% (n = 7562) heart rate data. More than 99% of body temperature, blood oxygen saturation, and heart rate data were within plausible ranges.
Repeat Infection Episodes
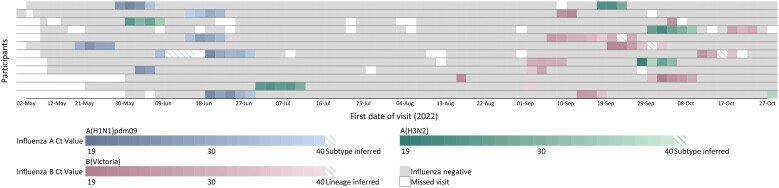
Sixty individuals (65%) had ≥1 episode of influenza during the 2022 season, of whom 48 (80%) had 1 episode, 11 (18%) had 2 episodes, and 1 (2%) had 3 episodes (Figure 1, Supplementary Table 3). Of the 73 infection episodes, 30 were influenza A(H1N1)pdm09, 13 influenza A(H3N2), 29 influenza B/Victoria, and 1 mixed (influenza A[H3N2] and B/Victoria). Most individuals with ≥1 episode in the same season (11/12, 92%) were infected with different types or subtypes, except for 1 who had 2 B/Victoria episodes (Figure 2).
Figure 1.
Influenza detection among children aged 6 to 23 months, ISiT study, South Africa, 2022: A, influenza A(H1N1)pdm09; B, influenza A (H3N2); C, influenza B Victoria. Columns are individual follow-up visits, and rows are individual participants. Follow-up visits are colored white if no sample was tested and light gray if the sample tested negative for influenza. For nasopharyngeal swabs that tested positive for influenza, follow-up visits are colored according to the influenza types and subtypes indicated in the legend. First and last columns are colored according to hemaglutination inhibition titer <40. ISiT, Identifying Symptoms in Toddlers.
Figure 2.
Influenza detection among children aged 6 to 23 months with repeat influenza infections, ISiT study, South Africa, 2022. Columns are individual follow-up visits, and rows are individual participants. Follow-up visits are colored white if no sample was tested and light gray if the sample tested negative for influenza. For nasopharyngeal swabs that tested positive for influenza, follow-up visits are colored according to the influenza types and subtypes indicated in the legend. ISiT, Identifying Symptoms in Toddlers.
Incidence
The incidence of infection, regardless of symptoms, was 86.6 per 100 person-seasons overall, 91.7 in infants aged 6 to 11 months, 82.6 in children aged 12 to 17 months, and 81.3 in children aged 18 to 23 months (Table 2). Boys and infants with mothers living with HIV had a higher incidence of infection (Table 1).
Table 2.
Incidence Rates of Influenza Infection and Illness by Age Group per 100 Person-Seasons, Klerksdorp, South Africa, 2022
| At Least 1 Episodea | Total Episodesb | Repeat Infections | ||||||
|---|---|---|---|---|---|---|---|---|
| Infections | Person-Seasons of Follow-up, number | No. | Rate (95% CI) | No. | Rate (95% CI) | No. (%) | ||
| Age, mo | ||||||||
| 6–11 | 39.3 | 30 | 76.4 (51.6–109.1) | 36 | 91.7 (64.2–127.0) | 6/36 (17) | ||
| 12–17 | 31.5 | 21 | 66.7 (41.3–102.0) | 26 | 82.6 (53.9–121.0) | 5/26 (19) | ||
| 18–23 | 13.5 | 9 | 66.5 (30.4–126.3) | 11 | 81.3 (40.6–145.5) | 2/11 (18) | ||
| All | 84.3 | 60 | 71.2 (54.3–91.7) | 73 | 86.6 (67.9–108.9) | 13/73 (18) | ||
| ≥1 Symptom | Fever | ILI (Fever and Cough) | Medically Attended Illness | |||||
|---|---|---|---|---|---|---|---|---|
| Illness | No.c | Rate (95% CI) | No.c | Rate (95% CI) | No.c | Rate (95% CI) | No.c | Rate (95% CI) |
| Age, mo | ||||||||
| 6–11 | 28 | 71.3 (47.4–103.1) | 15 | 38.2 (21.4–63.0) | 10 | 25.5 (12.2–46.9) | 3 | 7.6 (1.6–22.3) |
| 12–17 | 18 | 57.2 (33.9–90.4) | 15 | 47.7 (26.7–78.6) | 14 | 44.5 (24.3–74.6) | 1 | 3.2 (.1–17.7) |
| 18–23 | 9 | 66.5 (30.4–126.3) | 7 | 51.7 (20.8–106.6) | 5 | 37.0 (12.0–86.2) | 1 | 7.4 (.2–41.2) |
| All ages | 55 | 65.3 (49.2–85.0) | 37 | 43.9 (30.9–60.5) | 29 | 34.4 (23.1–49.4) | 5 | 5.9 (1.9–13.8) |
Incidence rate estimated as the number of episodes divided by the person-time under observation.
Abbreviation: ILI, influenza-like illness.
aIndividuals testing influenza positive at least once during follow-up counted once.
bIncludes repeat episodes.
cNumber of influenza infection episodes with ≥1 symptom, fever, ILI, or medically attended illness (includes repeat episodes).
Symptomatic Fraction
When all data from symptom diaries and nurse visits were combined, ≥1 symptom was reported from 75% (55/73) of episodes overall (Supplementary Tables 4 and 5). Fever was reported in 51% (37/73) of episodes and measured in 26% (19/73). There were 5 episodes of medically attended influenza-associated illness (5/73, 7%), 2 at a pharmacy and 3 at a public clinic. On multivariable analysis, the presence of ≥1 symptom was more frequently associated with influenza A (90%, 36/40) vs influenza B (47%, 9/19) infection and with longer shedding duration (≥7 days [89%, 32/36] vs <7 days [57%, 13/23]; Supplementary Table 6).
Per episode, caregivers' daily report of reported and measured fever was 19% (25/73, 34%) and 11% (15/73, 21%) higher than nurse-reported (11/73, 15%) and nurse-measured (7/73, 10%) fevers, respectively (Supplementary Table 4). Per episode, caregivers' daily report of any symptoms was 5% higher than nurse-reported symptoms (44/73 [60%] vs 40/73 [55%]).
Duration of Shedding
The median duration of shedding was 7 days overall (IQR, 4–9), which was similar for influenza A(H1N1)pdm09 (7 days; IQR, 5–9), A(H3N2) (9 days; IQR, 7–11; P = .134), and B/Victoria (7 days; IQR, 2–9; P = .732; Supplementary Figure 2). Influenza B infection was more frequently a single positive rRT-PCR result (7/12, 59%) than influenza A (4/36, 11%; P = .028). On multivariable analysis, low Ct value (proxy for higher viral load) and having ≥1 symptom were associated with shedding duration >7 days (Supplementary Table 7).
Correspondence of Serology and rRT-PCR
Baseline serology data were available for 91 participants. There were 12 (13%) with an HAI titer ≥40 for ≥1 type/subtype. There were no rRT-PCR–confirmed infections of the same type or subtype in these individuals. rRT-PCR and paired baseline–final visit serology specimens were available for 88 (95%) individuals, among whom there were 29 episodes of influenza A(H1N1)pdm09, 14 of influenza A(H3N2), and 31 of B/Victoria (Table 3, Supplementary Figure 3) with either rRT-PCR or serology. Considering serology as the reference test, for influenza A, rRT-PCR identified 100% of infections, while 3 infections (10%) with influenza B were not identified on rRT-PCR. Possible reasons for discordance between rRT-PCR results and serology are described in the footnotes to Table 3.
Table 3.
Correspondence of Influenza Episodes on PCR and Serology in 88 individuals with paired serology samples: Identifying Symptoms in Toddlers Study, South Africa 2022
| Influenza Episodes, No. (%) | |||
|---|---|---|---|
| Characteristic | A(H1N1)pdm09 | A(H3N2) | B Victoria |
| Episodes on | |||
| PCR or serology | 29 (100) | 14 (100) | 31 (100) |
| PCR | 29 (100) | 14 (100) | 28 (90) |
| Serologya | 29 (100) | 11 (79) | 25 (81) |
| PCR and serology | 29 (100) | 11 (79) | 22 (71) |
| Episodes identified on | |||
| PCR but not serology | 0 (0) | 3 (21)b | 6 (19)c |
| Serology but not PCR | 0 (0) | 0 (0) | 3 (10)d |
Abbreviation: PCR, polymerase chain reaction.
aFourfold rise in titer on hemaglutination inhibition.
bOf 3 individuals positive on PCR but not serology, the first positive PCR result was after the final blood draw for serology for 2, while the first positive PCR result was 8 days before the blood draw for 1, suggesting that there was not sufficient time for an immune response to occur.
cFor 3 episodes, the first positive PCR result was after the final blood draw for serology (of these, 2 were a second influenza infection). For the other 3 episodes, all had a blood draw >30 days after the start of the PCR-positive episode, but all were a single positive for influenza B (1 was a second influenza infection), suggesting a possible transient infection that did not elicit an immune response.
dFor all 3 episodes, the individual had an episode of influenza A infection in addition to the influenza B Victoria infection episode (2 A[H3N2] and 1 A[H1N1]pdm09); follow-up rates for these 3 children were 99%, 88%, and 78%.
Comparisons of Completeness and Concordance
A third weekly swab, in addition to twice-weekly nurse swabs, identified an additional 3 first episodes of influenza infection (2 weekly swabs had a sensitivity of 95% for influenza detection), with slightly higher sensitivity for detection of influenza A as compared with influenza B for nurse- and caregiver-collected swabs (Supplementary Table 8). Incidence of ≥1 influenza episode was 64.5 per 100 person-seasons according to 3 weekly swabs, as compared with 61.3 according to 2 weekly nurse-collected swabs (κ = 0.931). Incidence of ≥1 influenza infection was highest with rRT-PCR results of all swabs (nurse and caregiver), and the addition of serology did not increase the estimated incidence (Supplementary Table 9). rRT-PCR of nurse or caregiver swabs only or serology only each gave slightly lower estimates of incidence. Per visit, detection of influenza was similar for the first swab (nurse; 4.0%, 82/2031), second swab (nurse; 4.4%, 89/2043), and third swab (caregiver; 4.7%, 91/1954) collected each week (P > .4 for all comparisons).
DISCUSSION
In this cohort of frequently sampled young children, we describe a high incidence of 71.2 per 100 person-seasons for the first influenza episode, with ≥1 symptom reported in 75% of episodes. Caregiver-collected swabs were feasible and of good quality (human RNase P gene detected in 99%); however, the addition of a third weekly swab did not substantially increase the measured incidence. Caregiver diary completion was <70% but added >10% additional symptom data. We successfully followed up >94% of enrolled children for 6 months with >90% swab collection compliance. The high incidence of influenza and the ability to detect asymptomatic infections suggest that this platform could be suitable for future trials of vaccine efficacy and correlates of protection against infection and illness in children.
Our unique study design with frequent respiratory swab sampling and testing by rRT-PCR demonstrated very high rates of rRT-PCR–confirmed influenza infection in young children. The high incidence in our study was similar to those in a previous South African study, prior to the SARS-CoV-2 pandemic, with twice-weekly rRT-PCR testing, which found incidences of 66 and 68 per 100 person-seasons in children aged <1 and 1 to 4 years, respectively [4]. The SHIVERS cohort from Australia demonstrated an attack rate of 48% in children aged 0 to 4 years based on serology [10]. These rates are higher than those from early studies of serologically identified infection in the United States in young children [11]. Differences in attack rates may not only be attributed to the assay used but could be a result of differences in study design, population immunity, and contact characteristics as well as year-to-year variability in circulating influenza strains. Our intensive sampling strategy also allowed identification of children with repeat infections in the same season, with 20% of infected cases having ≥1 repeat infections, similar to our previous study [4].
Using a combination of daily symptom diaries and twice-weekly nurse interviews, we found a symptomatic fraction of 75%. This is similar to the symptomatic fractions identified in a previous South African cohort study, at 79% and 73% in children aged <1 and 1 to 4 years, respectively [4]. Systematic reviews of the proportion of influenza infections with symptoms have identified heterogeneity in estimates for different study designs (outbreak investigations [4%–28% vs serologic studies 65%–85%) but estimates are rarely presented by age group and studies of similar design to ours are lacking 12, 13]. In studies where data by age group are available, symptomatic fractions are generally higher in children than in adults and adolescents [11, 14, 15]. Overall rates of reported symptoms were similar on twice-weekly nurse visits (55%) vs daily symptom diaries (60%), as were rates of cough (41% vs 45%). However, fever was reported at substantially higher rates in symptom diaries than at nurse visits. In young children, fever is a well-established marker of severity for influenza, while other severity markers (eg, admission) are not easy to assess because they occur infrequently and the probability of admission differs by community [1]. Robust identification of measured fever will be important for future studies of vaccine efficacy and correlates of protection against infection and illness; therefore, attention should be paid to strategies for improving and maintaining compliance with symptom diary completion, as well as evaluation of wearable devices [16].
The median duration of shedding was 7 days in our study and did not vary by influenza type or subtype. Similar to previous studies, we found that shedding duration was longer in participants who were symptomatic and those with a low Ct value on rRT-PCR (proxy for higher viral load) [4]. Symptoms were also more common in those with shedding for >7 days. This suggests a strong association between presence of symptoms, longer duration of shedding, and higher viral load, which has also been identified for other respiratory viruses, such as respiratory syncytial virus and SARS-CoV-2 [6, 17]. Future clinical trials of influenza vaccine with platforms such as ours could quantify reductions in shedding duration and Ct value in vaccinated individuals.
Our study aimed to evaluate the additional benefit of a third weekly swab, as well as to pilot the feasibility of caregiver-collected swabs, as an alternative to nurse-collected swabs, for identification of influenza infections. We achieved good compliance with caregiver swabs. Human RNase P was present in 99% (2032/2045) of caregiver-collected swabs, indicating good sample quality, and the influenza detection rate was similar for caregiver- and nurse-collected swabs. A third weekly swab on the weekend did not substantially increase incidence, with only 3 additional first influenza episodes identified. This suggests that twice-weekly swabs are likely sufficient to identify the majority of rRT-PCR–confirmed infection episodes in this age group and demonstrates that caregiver swabs are feasible, accurate, and likely more affordable. It is possible that a third weekly swab would have more benefit in older persons where the duration of viral shedding is shorter [4].
The incidence was similar with rRT-PCR and paired serology, suggesting that our intensive sampling approach identified the majority of influenza infections on rRT-PCR. While burden estimates were similar between these approaches, repeated swabbing has the added benefit of providing information on the duration of shedding, determining Ct values, and identifying repeat infections. Serologic sampling may not be necessary for infection ascertainment if resources are limited; however, serologic sampling allows examination of correlates of protection. Most discordant results could be explained by the timing of sample collection. There were 3 episodes with a single positive rRT-PCR result for influenza B/Victoria not associated with seroconversion, suggesting possible transient infection that did not elicit an immune response. On entry to the study, a small number of children (n = 12) had HAI titers higher than 1/40 for any type or subtype, but interestingly there were no breakthrough infections of the homologous type in any of these individuals.
Our study had limitations. Caregivers may have varied in their propensity to identify symptoms, and our sample may not have been representative of the general population. Exclusion of children with self-limiting respiratory illness at baseline may have biased our results, and future studies may consider rescreening 2 weeks later. Our study was conducted in the year after the COVID-19 pandemic ended, which could have been an atypical season, but according to surveillance data, in 2022 influenza epidemiology had returned to prepandemic patterns in South Africa. Incidence and symptomatic fractions were similar to prepandemic studies, suggesting that the 2022 season may have been representative of postpandemic influenza epidemiology [4, 18].
Given our experiences in this study, we recommend that future studies include twice-weekly nasal swabbing irrespective of symptoms, with caregiver collection of 1 swab to reduce costs. Symptom ascertainment should be through a combination of symptom diaries and nurse visits with daily temperature measurement. Daily pulse oximetry and heart rate measurement should not be performed. Classic case definitions can be applied at the analysis stage to allow comparison with other studies.
To support the effort to develop improved influenza vaccines, platforms are needed that are suitable to evaluate (1) the efficacy of current and new influenza vaccines against infection vs illness and (2) the duration of shedding for breakthrough and repeat infections in vaccinated individuals [19, 20]. Such platforms could also provide important information on correlates of protection against infection and illness. We have demonstrated that our approach with intensive rRT-PCR sampling identifies high rates of PCR-confirmed infection with robust symptom ascertainment, suggesting possible application for future clinical trials. Caregiver swabbing was effective and twice-weekly swabs identified most infections. Future studies should focus on improved approaches for caregiver symptom diary collection, as this is key to robust symptom data collection.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Cheryl Cohen, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Mignon du Plessis, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Neil Martinson, Perinatal HIV Research Unit, MRC Soweto Matlosana Collaborating Centre for HIV/AIDS and TB, University of the Witwatersrand, Johannesburg, South Africa; Center for TB Research, Johns Hopkins University, Baltimore, Maryland.
Jocelyn Moyes, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases.
Sibongile Walaza, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Nicole Wolter, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Mvuyo Makhasi, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Fahima Moosa, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Myrna Charles, Global Influenza Branch, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Aaron M Samuels, Global Influenza Branch, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia; Influenza Program, Centers for Disease Control and Prevention, Pretoria, South Africa.
Stefano Tempia, Influenza Program, Centers for Disease Control and Prevention, Pretoria, South Africa.
Tumelo Moloantoa, Perinatal HIV Research Unit, MRC Soweto Matlosana Collaborating Centre for HIV/AIDS and TB, University of the Witwatersrand, Johannesburg, South Africa.
Bekiwe Ncwana, Perinatal HIV Research Unit, MRC Soweto Matlosana Collaborating Centre for HIV/AIDS and TB, University of the Witwatersrand, Johannesburg, South Africa.
Louisa Phalatse, Perinatal HIV Research Unit, MRC Soweto Matlosana Collaborating Centre for HIV/AIDS and TB, University of the Witwatersrand, Johannesburg, South Africa.
Amelia Buys, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases.
Alicia Fry, Epidemiology and Prevention Branch, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Eduardo Azziz Baumgartner, Global Influenza Branch, Influenza Division, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia.
Anne von Gottberg, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Jackie Kleynhans, Centre for Respiratory Diseases and Meningitis, National Institute for Communicable Diseases; School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Notes
Acknowledgments . The authors thank all the individuals who kindly agreed to participate in the study as well as the many field and laboratory staff who worked tirelessly to implement the study.
Author contributions. Conception and design of study: C. C., N. M., J. M., ST, AF, EAB, A. v. G., J. K. Data collection and laboratory processing: C. C., M. d. P., N. M., J. M., S. W., N. W., M. M., F. M., S. T., T. M., B. N., L. P., A. B., A. v. G., J. K. Analysis and interpretation: C. C., M. d. P., N. M., J. M., S. W., N. W., M. M., F. M., M. C., A. M. S., S. T., T. M., B. N., L. P., A. B., A. F., E. A. B., A. v. G., J. K. C. C. and J. K. accessed and verified the underlying data. C. C. and J. K. drafted the article. All authors critically reviewed the article. All authors had access to all the data reported in the study.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
Ethical approval statement . The ISiT protocol was approved by the University of Witwatersrand Human Research Ethics Committee (Medical; reference 220104). Written informed consent was obtained from all caregivers prior to data collection. Participants received grocery store vouchers of US $3 per nurse visit and an additional US $3 following the weekly parent-collected swab to compensate for time required for specimen and data collection. After 28 August, caregivers were incentivized with an additional US $1.50 or $3 if >50% or >80% of weekly symptom diaries were completed, respectively. Caregivers were issued a study electronic tablet, which they retained at the end of the study. Study mobile data were provided through direct credit to mobile numbers.
Financial support . This work was supported by the Centers for Disease Control and Prevention USA (cooperative agreement 1U01IP001048).
References
- 1. Ferdinands JM, Thompson MG, Blanton L, Spencer S, Grant L, Fry AM. Does influenza vaccination attenuate the severity of breakthrough infections? A narrative review and recommendations for further research. Vaccine 2021; 39:3678–95. [DOI] [PubMed] [Google Scholar]
- 2. Tempia S, Moyes J, Cohen AL, et al. Health and economic burden of influenza-associated illness in South Africa, 2013–2015. Influenza Other Respir Viruses 2019; 13:484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cohen C, McMorrow ML, Martinson NA, et al. Cohort profile: a prospective household cohort study of influenza, respiratory syncytial virus and other respiratory pathogens community burden and transmission dynamics in South Africa, 2016–2018. Influenza Other Respir Viruses 2021; 15:789–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen C, Kleynhans J, Moyes J, et al. Asymptomatic transmission and high community burden of seasonal influenza in an urban and a rural community in South Africa, 2017–18 (PHIRST): a population cohort study. Lancet Glob Heal 2021; 9:e863–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blumberg LH, Cohen C, Dawood H, et al. Influenza: NICD recommendations for the diagnosis, management, prevention and public health response. 2023. Available at: https://www.google.co.za/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwj1wtDBo6mAAxXGTEEAHThVCl8QFnoECBwQAQ&url=https%3A%2F%2Fwww.nicd.ac.za%2Fwp-content%2Fuploads%2F2023%2F05%2FInfluenza-guidelines_-25April-2023-final.pdf&usg=AOvVaw1kkaZ_3WoJlNzDbRC. Accessed date 1 June 2024.
- 6. Cohen C, Kleynhans J, von Gottberg A, et al. SARS-CoV-2 incidence, transmission, and reinfection in a rural and an urban setting: results of the PHIRST-C cohort study, South Africa, 2020–21. Lancet Infect Dis 2022; 22:821–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 1999; 37:937–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2020; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang QS, Bandaranayake D, Wood T, et al. Risk factors and attack rates of seasonal influenza infection : results of the Southern Hemisphere Influenza and Vaccine Effectiveness Research and Surveillance (SHIVERS). Seroepidemiologic Cohort Study 2019; 219:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monto AS, Koopman JS, Longini IM. Tecumseh study of illness: XIII. Influenza infection and disease, 1976–1981. Am J Epidemiol 1985; 121:811–22. [DOI] [PubMed] [Google Scholar]
- 12. Furuya-Kanamori L, Cox M, Milinovich GJ, Magalhaes RJ, Mackay IM, Yakob L. Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerg Infect Dis 2016; 22:1052–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leung NH, Xu C, Ip DK, Cowling BJ. Review article: the fraction of influenza virus infections that are asymptomatic—a systematic review and meta-analysis. Epidemiology 2015; 26:862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fitzner J, Qasmieh S, Mounts AW, et al. Revision of clinical case definitions: influenza-like illness and severe acute respiratory infection. Bull World Health Organ 2018; 96:122–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Suess T, Remschmidt C, Schink SB, et al. Comparison of shedding characteristics of seasonal influenza virus (sub)types and influenza A(H1N1)pdm09; Germany, 2007–2011. PLoS One 2012; 7:e51653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ginsburg GS, Picard RW, Friend SH. Key issues as wearable digital health technologies enter clinical care. N Engl J Med 2024; 390:1118–27. [DOI] [PubMed] [Google Scholar]
- 17. Cohen C, Kleynhans J, Moyes J, et al. Incidence and transmission of respiratory syncytial virus in urban and rural South Africa, 2017–2018. Nat Commun 2024; 15:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolter N, Buys A, Walaza S, et al. Influenza surveillance in South Africa: weeks 1–31, 2022. Public Heal Surveill Bull 2022; 20:76–99. [Google Scholar]
- 19. Kennedy-Shaffer L, Kahn R, Lipsitch M. Estimating vaccine efficacy against transmission via effect on viral load. Epidemiology 2021; 32:820–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lipsitch M, Kahn R. Interpreting vaccine efficacy trial results for infection and transmission. Vaccine 2021; 39:4082–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.