Abstract
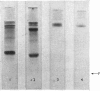
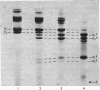
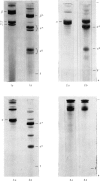
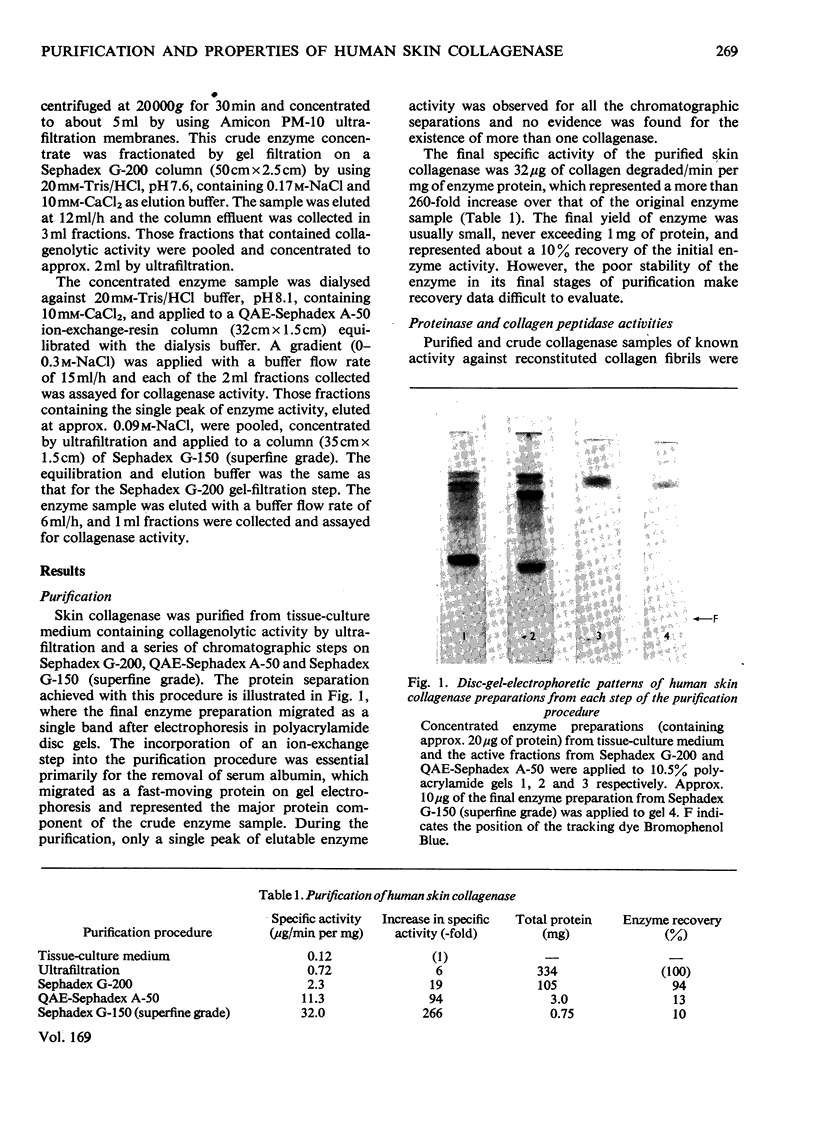
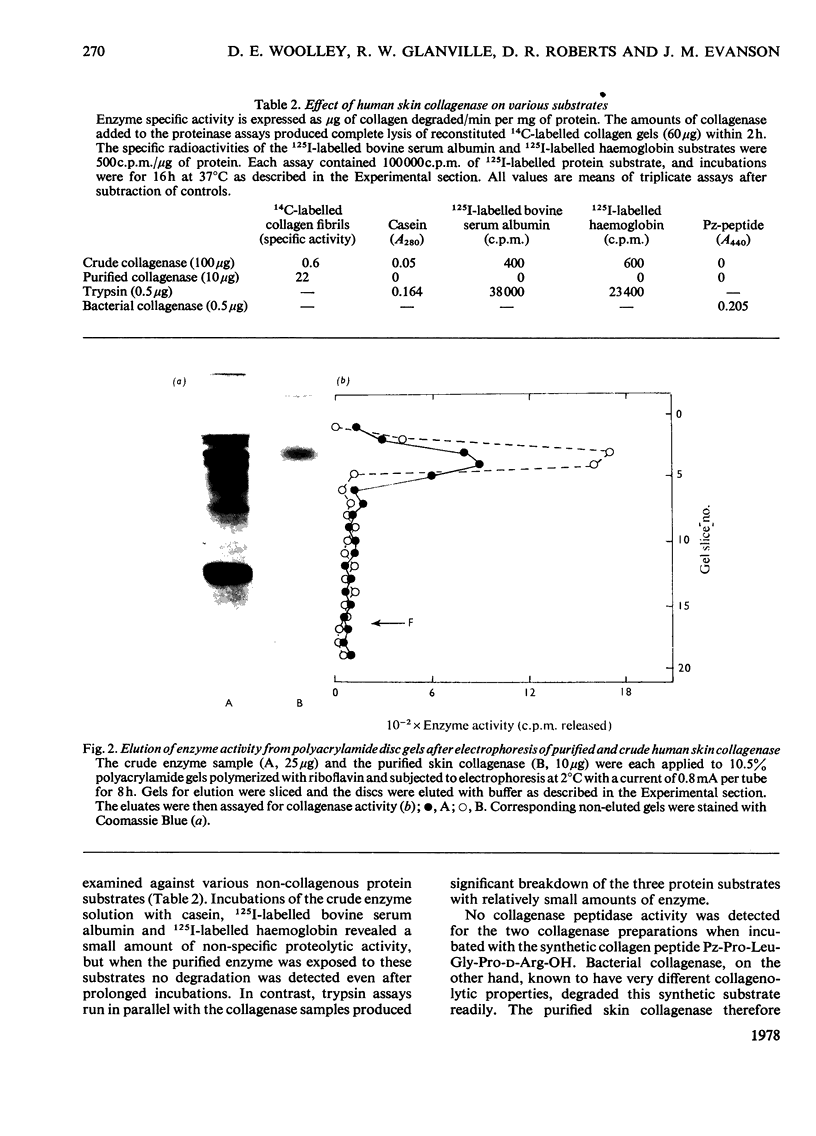
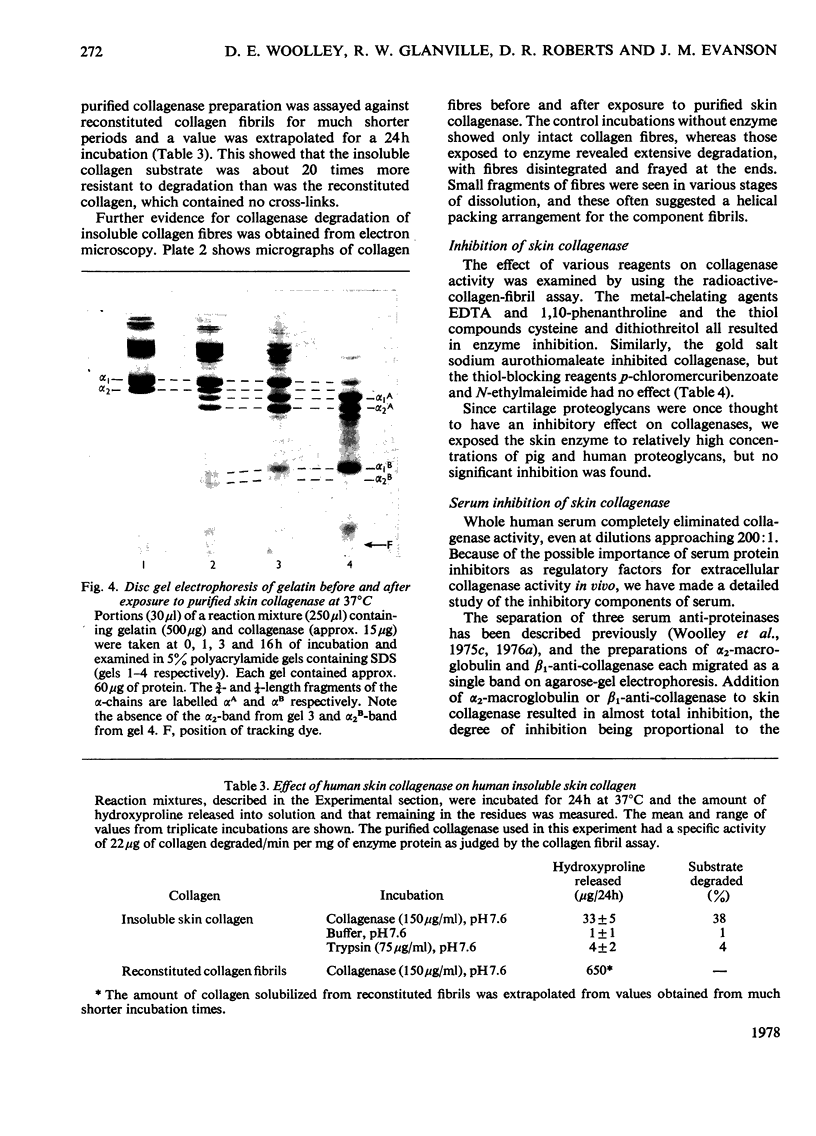
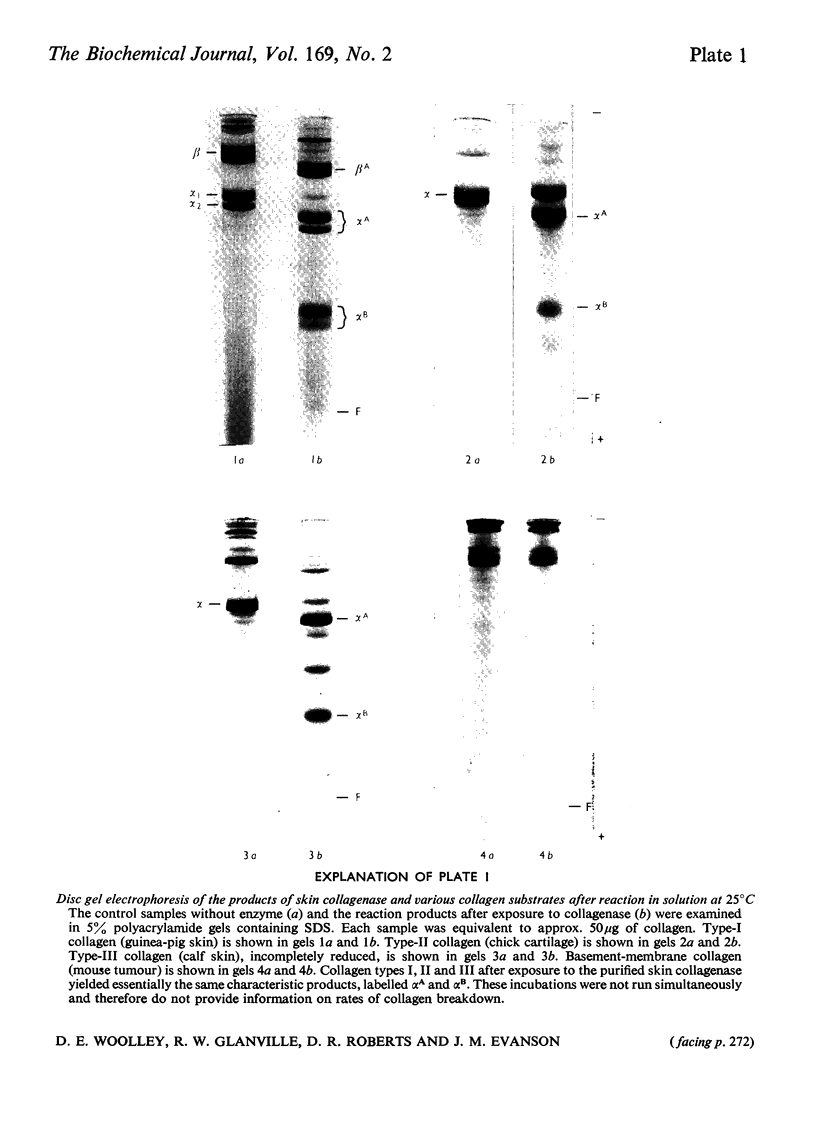
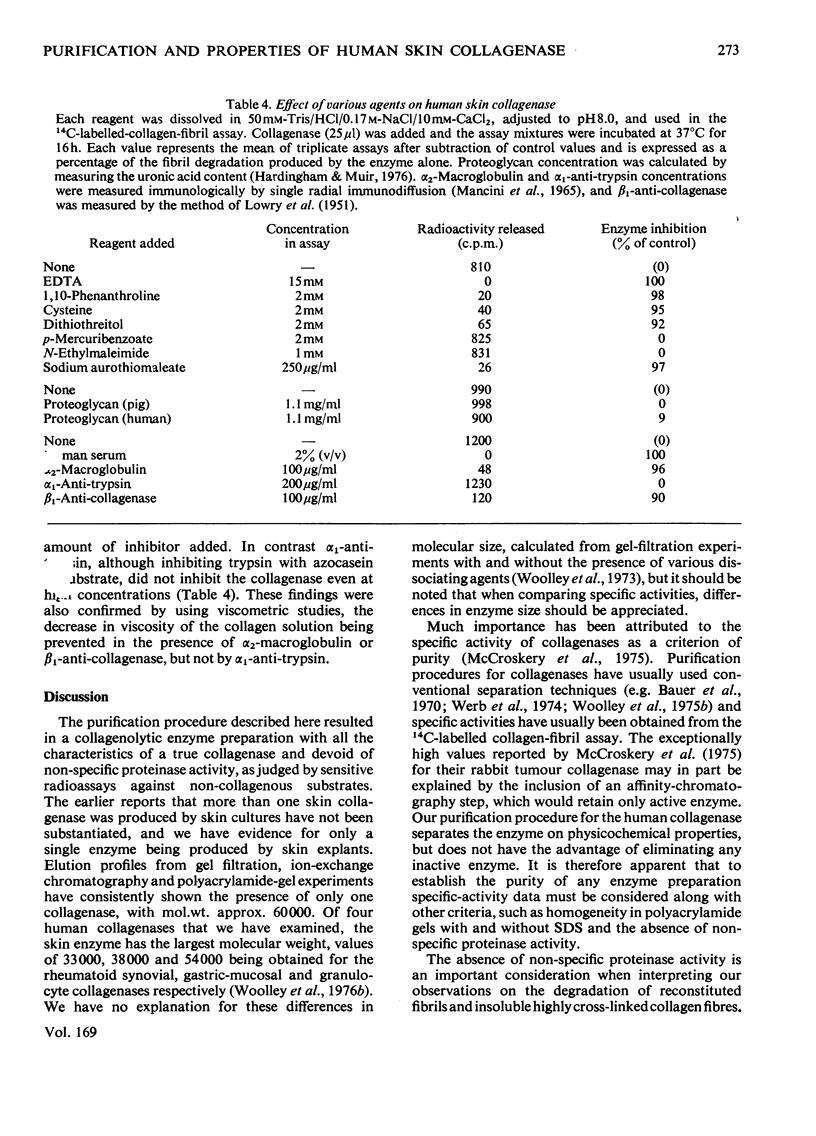
1. The neutral collagenase released into the culture medium by explants of human skin tissue was purified by ultrafiltration and column chromatography. The final enzyme preparation had a specific activity against thermally reconstituted collagen fibrils of 32μg of collagen degraded/min per mg of enzyme protein, representing a 266-fold increase over that of the culture medium. Electrophoresis in polyacrylamide disc gels showed it to migrate as a single protein band from which enzyme activity could be eluted. Chromatographic and polyacrylamide-gel-elution experiments provided no evidence for the existence of more than one active collagenase. 2. The molecular weight of the enzyme estimated from gel filtration and sodium dodecyl sulphate/polyacrylamide-gel electrophoresis was approx. 60000. The purified collagenase, having a pH optimum of 7.5–8.5, did not hydrolyse the synthetic collagen peptide 4-phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-d-Arg-OH and had no non-specific proteinase activity when examined against non-collagenous proteins. 3. It attacked undenatured collagen in solution at 25°C, producing the two characteristic products TCA(¾) and TCB(¼). Collagen types I, II and III were all cleaved in a similar manner by the enzyme at 25°C, but under similar conditions basement-membrane collagen appeared not to be susceptible to collagenase attack. At 37°C the enzyme attacked gelatin, producing initially three-quarter and one-quarter fragments of the α-chains, which were degraded further at a lower rate. As judged by the release of soluble hydroxyproline peptides and electron microscopy, the purified enzyme degraded insoluble collagen derived from human skin at 37°C, but at a rate much lower than that for reconstituted collagen fibrils. 4. Inhibition of the skin collagenase was obtained with EDTA, 1,10-phenanthroline, cysteine, dithiothreitol and sodium aurothiomaleate. Cartilage proteoglycans did not inhibit the enzyme. The serum proteins α2-macroglobulin and β1-anti-collagenase both inhibited the enzyme, but α1-anti-trypsin did not. 5. The physicochemical and enzymic properties of the skin enzyme are discussed in relation to those of other human collagenases.
Full text
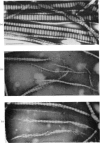
PDF




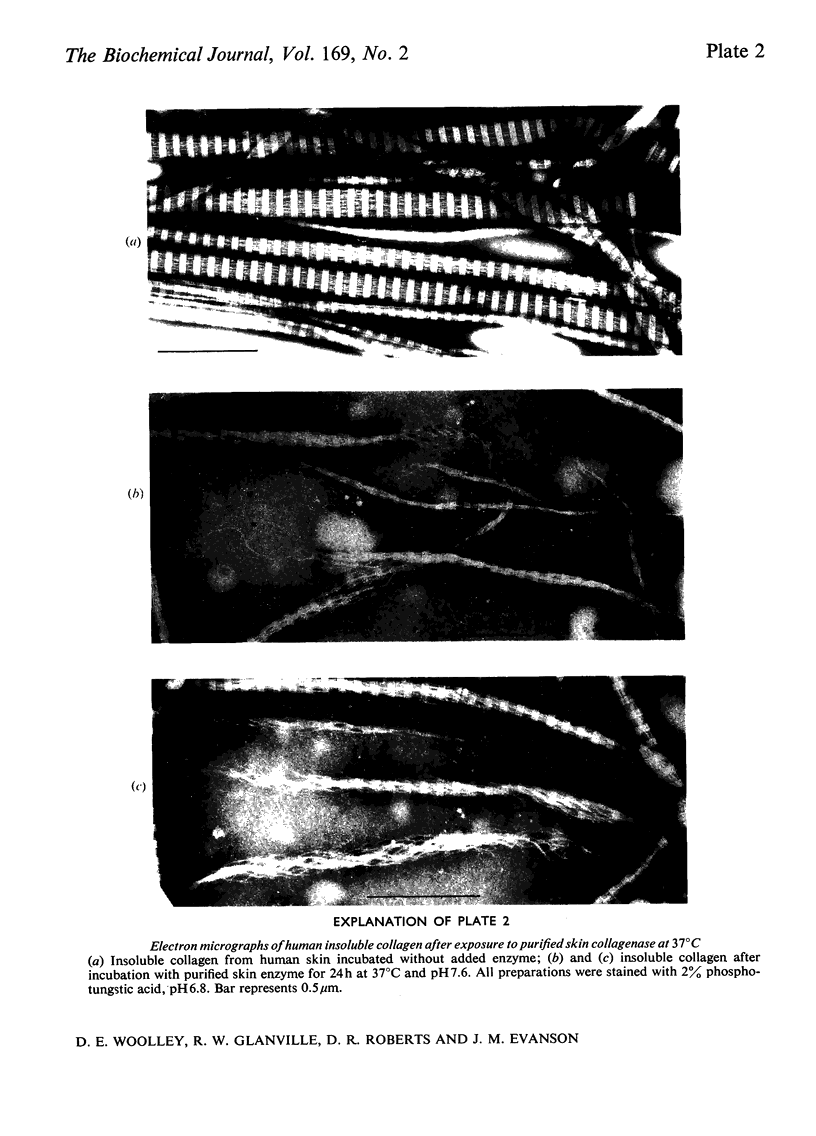



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer E. A., Eisen A. Z., Jeffrey J. J. Immunologic relationship of a purified human skin collagenase to other human and animal collagenases. Biochim Biophys Acta. 1970 Apr 22;206(1):152–160. doi: 10.1016/0005-2744(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Eisen A. Z., Jeffrey J. J. Radioimmunoassay of human collagenase. Specificity of the assay and quantitative determination of in vivo and in vitro human skin collagenase. J Biol Chem. 1972 Oct 25;247(20):6679–6685. [PubMed] [Google Scholar]
- Bauer E. A., Eisen A. Z., Jeffrey J. J. Regulation of vertebrate collagenase activity in vivo and in vitro. J Invest Dermatol. 1972 Jul;59(1):50–55. doi: 10.1111/1523-1747.ep12625767. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Jeffrey J. J., Eisen A. Z. Preparation of three vertebrate collagenases in pure form. Biochem Biophys Res Commun. 1971 Aug 20;44(4):813–818. doi: 10.1016/0006-291x(71)90783-2. [DOI] [PubMed] [Google Scholar]
- Bauer E. A., Stricklin G. P., Jeffrey J. J., Eisen A. Z. Collagenase production by human skin fibroblasts. Biochem Biophys Res Commun. 1975 May 5;64(1):232–240. doi: 10.1016/0006-291x(75)90243-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Bloch K. J., Sakai T. Inhibition of human skin collagenase by human serum. J Lab Clin Med. 1970 Feb;75(2):258–263. [PubMed] [Google Scholar]
- Eisen A. Z. Human skin collagenase: localization and distribution in normal human skin. J Invest Dermatol. 1969 May;52(5):442–448. doi: 10.1038/jid.1969.76. [DOI] [PubMed] [Google Scholar]
- Eisen A. Z., Jeffrey J. J., Gross J. Human skin collagenase. Isolation and mechanism of attack on the collagen molecule. Biochim Biophys Acta. 1968 Mar 25;151(3):637–645. doi: 10.1016/0005-2744(68)90010-7. [DOI] [PubMed] [Google Scholar]
- Fullmer H. M., Gibson W. A., Lazarus G., Stam A. C., Jr Collagenolytic activity of the skin associated with neuromuscular diseases including amyotrophic lateral sclerosis. Lancet. 1966 May 7;1(7445):1007–1009. doi: 10.1016/s0140-6736(66)90116-4. [DOI] [PubMed] [Google Scholar]
- GRANT R. A. ESTIMATION OF HYDROXYPROLINE BY THE AUTOANALYSER. J Clin Pathol. 1964 Nov;17:685–686. doi: 10.1136/jcp.17.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J., Harper E., Harris E. D., McCroskery P. A., Highberger J. H., Corbett C., Kang A. H. Animal collagenases: specificity of action, and structures of the substrate cleavage site. Biochem Biophys Res Commun. 1974 Nov 27;61(2):605–612. doi: 10.1016/0006-291x(74)91000-6. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Farrell M. E. Resistance to collagenase: a characteristic of collagen fibrils cross-linked by formaldehyde. Biochim Biophys Acta. 1972 Aug 31;278(1):133–141. doi: 10.1016/0005-2795(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (first of three parts). N Engl J Med. 1974 Sep 12;291(11):557–563. doi: 10.1056/NEJM197409122911105. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (second of three parts). N Engl J Med. 1974 Sep 19;291(12):605–609. doi: 10.1056/NEJM197409192911205. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. Collagenases (third of three parts). N Engl J Med. 1974 Sep 26;291(13):652–661. doi: 10.1056/NEJM197409262911305. [DOI] [PubMed] [Google Scholar]
- Kuettner K. E., Hiti J., Eisenstein R., Harper E. Collagenase inhibition by cationic proteins derived from cartilage and aorta. Biochem Biophys Res Commun. 1976 Sep 7;72(1):40–46. doi: 10.1016/0006-291x(76)90957-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leibovich S. J., Weiss J. B. Elucidation of the exact sites of cleavage of tropocollagen by rheumatoid synovial collagenase: correlation of cleavage sites with fibril structure. Connect Tissue Res. 1973;2(1):11–19. doi: 10.3109/03008207309152595. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- McCroskery P. A., Richards J. F., Harris E. D., Jr Purification and characterization of a collagenase extracted from rabbit tumours. Biochem J. 1975 Oct;152(1):131–142. doi: 10.1042/bj1520131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. J., Harris E. D., Jr, Chung E., Finch J. E., Jr, McCroskery P. A., Butler W. T. Cleavage of Type II and III collagens with mammalian collagenase: site of cleavage and primary structure at the NH2-terminal portion of the smaller fragment released from both collagens. Biochemistry. 1976 Feb 24;15(4):787–792. doi: 10.1021/bi00649a009. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Orkin R. W., Gehron P., McGoodwin E. B., Martin G. R., Valentine T., Swarm R. A murine tumor producing a matrix of basement membrane. J Exp Med. 1977 Jan 1;145(1):204–220. doi: 10.1084/jem.145.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROCKOP D. J., UDENFRIEND S. A specific method for the analysis of hydroxyproline in tissues and urine. Anal Biochem. 1960 Nov;1:228–239. doi: 10.1016/0003-2697(60)90050-6. [DOI] [PubMed] [Google Scholar]
- Sellers A., Cartwright E., Murphy G., Reynolds J. J. Evidence that latent collagenases are enzyme-inhibitor complexes. Biochem J. 1977 May 1;163(2):303–307. doi: 10.1042/bj1630303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shinkai H., Kawamoto T., Hori H., Nagai Y. A complex of collagenase with low molecular weight inhibitors in the culture medium of embryonic chick skin explants. J Biochem. 1977 Jan;81(1):261–263. doi: 10.1093/oxfordjournals.jbchem.a131444. [DOI] [PubMed] [Google Scholar]
- Sopata I., Dancewicz A. M. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim Biophys Acta. 1974 Dec 29;370(2):510–523. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Jackson D. S. Purification and amino acid composition of monomeric and polymeric collagens. Biochem J. 1967 Aug;104(2):534–536. doi: 10.1042/bj1040534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricklin G. P., Bauer E. A., Jeffrey J. J., Eisen A. Z. Human skin collagenase: isolation of precursor and active forms from both fibroblast and organ cultures. Biochemistry. 1977 Apr 19;16(8):1607–1615. doi: 10.1021/bi00627a013. [DOI] [PubMed] [Google Scholar]
- Timpl R., Glanville R. W., Nowack H., Wiedemann H., Fietzek P. P., Kühn K. Isolation, chemical and electron microscopical characterization of neutral-salt-soluble type III collagen and procollagen from fetal bovine skin. Hoppe Seylers Z Physiol Chem. 1975 Nov;356(11):1783–1792. doi: 10.1515/bchm2.1975.356.2.1783. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- WUENSCH E., HEIDRICH H. G. ZUR QUANTITATIVEN BESTIMMUNG DER KOLLAGENASE. Hoppe Seylers Z Physiol Chem. 1963;333:149–151. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiss J. B. Enzymic degradation of collagen. Int Rev Connect Tissue Res. 1976;7:101–157. doi: 10.1016/b978-0-12-363707-9.50009-5. [DOI] [PubMed] [Google Scholar]
- Werb Z., Burleigh M. C., Barrett A. J., Starkey P. M. The interaction of alpha2-macroglobulin with proteinases. Binding and inhibition of mammalian collagenases and other metal proteinases. Biochem J. 1974 May;139(2):359–368. doi: 10.1042/bj1390359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley D. E., Evanson J. M. Collagenase and its natural inhibitors in relation to the rheumatoid joint. Connect Tissue Res. 1977;5(1):31–35. doi: 10.3109/03008207709152609. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Crossley M. J., Evanson J. M. Purification of rheumatoid synovial collagenase and its action on soluble and insoluble collagen. Eur J Biochem. 1975 Jun;54(2):611–622. doi: 10.1111/j.1432-1033.1975.tb04173.x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Glanville R. W., Evanson J. M. Differences in the physical properties of collagenases isolated from rheumatoid synovium and human skin. Biochem Biophys Res Commun. 1973 Apr 2;51(3):729–734. doi: 10.1016/0006-291x(73)91376-4. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Lindberg K. A., Glanville R. W., Evanson J. M. Action of rheumatoid synovial collagenase on cartilage collagen. Different susceptibilities of cartilage and tendon collagen to collagenase attack. Eur J Biochem. 1975 Jan 2;50(2):437–444. doi: 10.1111/j.1432-1033.1975.tb09821.x. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Roberts D. R., Evanson J. M. Inhibition of human collagenase activity by a small molecular weight serum protein. Biochem Biophys Res Commun. 1975 Sep 16;66(2):747–754. doi: 10.1016/0006-291x(75)90573-2. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Roberts D. R., Evanson J. M. Small molecular weight beta 1 serum protein which specifically inhibits human collagenases. Nature. 1976 May 27;261(5558):325–327. doi: 10.1038/261325a0. [DOI] [PubMed] [Google Scholar]
- Woolley D. E., Tucker J. S., Green G., Evanson J. M. A neutral collagenase from human gastric mucosa. Biochem J. 1976 Jan 1;153(1):119–126. doi: 10.1042/bj1530119. [DOI] [PMC free article] [PubMed] [Google Scholar]