Abstract
Background
EDP-938 is an oral once-daily RSV nucleoprotein (N) inhibitor with potent antiviral activity. In a human RSV challenge trial, EDP-938 significantly reduced viral load and symptom severity. During antiviral development, it is critical to understand the propensity for resistance to develop. In vitro studies of EDP-938 suggest a higher barrier to resistance as compared to RSV fusion inhibitors. We evaluated the development of viral resistance to EDP-938 in a human challenge trial.
Methods
A subset of the 124 participants with RSV infection were chosen for genetic analysis; 159 nasal wash samples from 48 participants were analyzed using next-generation sequencing of the N gene of RSV. Of the 48 participant sampled, 37 were from EDP-938–treated and 11 were placebo-treated participants, representing 45% and 26% of the participants, respectively. The effects of treatment-emergent mutations on viral load, EDP-938 efficacy, and viral fitness were evaluated.
Results
Two of the 37 EDP-938–treated participants with samples sequenced had treatment-emergent mutations: N:L139I and N:E112G. From in vitro analysis, N:L139I reduced sensitivity to EDP-938 by approximately 10-fold, while N:E112G had no effect. However, N:L139I was associated with a reduction in viral fitness, suggesting clinical resistance is associated with fitness costs. Neither of these variants were associated with reduced viral clearance.
Conclusions
In human RSV infections treated with EDP-938, emergence of RSV variants with reduced sensitivity to EDP-938 occurred in only 1 participant and was associated with reduced viral fitness. EDP-938's high barrier to resistance highlights its robust mechanism of action.
Clinical Trials Registration
Keywords: RSV, antiviral, antiviral resistance, clinical trials, challenge trial, N inhibitor, EDP-938
In an RSV human challenge trial only 1 participant had a treatment-emergent mutation associated with resistance to EDP-938. This resistance mutation was associated with reductions in viral fitness. Thus, EDP-938 has a high barrier to resistance.
Respiratory syncytial virus (RSV) is a ubiquitous pathogen that infects the respiratory tract. For young children, the elderly, and those with underlying medical conditions, RSV can result in substantial morbidity and mortality [1–4]. While there are prophylactic monoclonal antibodies and vaccines approved for the prevention of RSV [5–7], there is no specific treatment for RSV infection. Aerosolized ribavirin has been used to treat severe RSV infection, but it is not recommended by the American Academy of Pediatrics due to its unclear therapeutic benefits and concerns about teratogenicity and toxicity [8].
EDP-938 is an orally bioavailable RSV nucleoprotein (N) inhibitor currently being studied in 2 phase 2b clinical trials (NCT04816721, NCT05568706). EDP-938 displays potent anti-RSV activity in vitro and was efficacious in a nonhuman primate model of RSV infection [9]. In phase 1 clinical trials, EDP-938 was safe and generally well tolerated [10], prompting its entry into a phase 2a human challenge trial (NCT03691623). Healthy volunteers were inoculated with RSV-A M37b and randomly assigned to an EDP-938 dosing group or placebo cohort. The study was conducted in 2 independent parts. In the first, 115 participants received either EDP-938 at 600 mg once daily, 300 mg twice daily after a 500-mg loading dose, or placebo. In the second part, 63 participants received 300 mg once daily after a 600-mg loading dose, 200 mg twice daily after a 400-mg loading dose, or placebo. In all dosing groups, EDP-938 significantly reduced viral load and symptom score as compared to placebo [11].
A major concern with direct acting antivirals (DAAs) is the development of resistance. Indeed, experimental generation of in vitro resistance to RSV antivirals targeting the fusion protein resulted in generation of fusion protein mutations upon initial exposure of 10-fold the 50% maximum effective concentration (EC50) that mediated >1000-fold decreases in potency with minimal effect on viral fitness [12–17]. Resistance to one fusion inhibitor has, to date, mediated cross-resistance to other fusion inhibitors, which is not unexpected because many of them bind in the same region of the fusion protein [14, 18, 19]. The rapid emergence of fusion inhibitor resistance has also been observed in both a human challenge trial and naturally occurring infection [15, 17]. In contrast, the barrier to in vitro resistance for EDP-938, a replication inhibitor, is high, as demonstrated by extensive passaging (approximately 10 generations) below 10-fold of the EC50 needed to generate resistant mutants [9]. Much smaller potency shifts (≤70-fold) were observed for EDP-938–resistant mutants with an inverse relationship between resistance and viral fitness [9]. These data suggest that the barrier to clinical resistance is higher for EDP-938 as compared to fusion inhibitors.
To determine the propensity for EDP-938–resistant viruses to emerge in a clinical setting, we performed next-generation sequencing (NGS) of the RSV N gene on nasal swabs from participants enrolled in a human challenge trial. We found only 1 EDP-938–treated participant with a mutation that confers resistance. Thus, EDP-938's barrier to resistance in a clinical setting is relatively high compared to fusion inhibitors, providing a distinct advantage of its mechanism of action.
METHODS
Clinical Study Design and Sample Collection
EDP-938 efficacy and safety was assessed in a randomized, double-blind, placebo-controlled human challenge trial (NCT03691623) [11], conducted by hVivo. Nasal wash samples were collected 1 day prior to challenge, and then twice daily from 2 days after challenge until the morning of study day 12, for a total of 21 nasal washes postchallenge. Upon positive detection of RSV by qualitative polymerase chain reaction (PCR; 3M Integrated Cycle; Focus Diagnostics) or after day 5, participants were randomized into a treatment group. All participants provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki (1996 version), the International Conference on Harmonization Good Clinical Practice guidelines.
Plasmids
The RSV reverse genetics plasmid system was obtained from Dr Marty Moore [20]. Site-directed mutagenesis was performed using NEB Q5 on a shuttle vector containing the N open reading frame (ORF) between the AvrII and BstBI sites. Upon sequence confirmation of the mutation, the N ORF region was PCR amplified and gel purified using the Macherey Nagel NucleoSpin Gel and PCR Clean-Up Mini Kit. The pSynkRSV-I19F bacterial artificial chromosome (BAC) was digested with AvrII and BstBI and gel purified using the Macherey Nagel NucleoSpin Gel and PCR Clean-Up Mini Kit. The PCR amplified regions were then inserted into the AvrII and BstBI site of pSynkRSV-F:I19F using HiFi assembly mix (NEB). All primers are listed in Supplementary Table 1.
Cell Lines
HEp-2 cells (CCL-23) and Vero cells (CCL-81) were obtained from American Type Culture Collection. HEp-2 cells were cultured and infected in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (HyClone), 1% penicillin/streptomycin (Life Technologies), and 1% minimal essential media nonessential amino acids (Life Technologies). Vero cells were cultured in and infected in minimum essential medium (MEM; Life Technologies) supplemented with 10% fetal bovine serum (HyClone). All cell lines were maintained in and all infections were performed at 37°C in 5% CO2 atmosphere.
Chemical Inhibitors
AK0529 (Ziresovir) and RV521 (Sisunatovir) were purchased from MedChemExpress. All other compounds were synthesized by Enanta Pharmaceuticals, Inc, Watertown, MA.
Viruses
Preparation and recovery of recombinant wild-type and mutant RSV was performed using the BAC-based system described in Hotard et al [20] with the following modifications. The wild-type (WT) or mutant versions of pSynkRSV-I19F along with the 4 helper plasmids and pCDNA3.1-T7 were transfected into Vero cells using Lipofectamine 2000CD (Life Technologies) following the manufacturer's instructions. Cells were expanded and the media was replaced every 2–3 days until the observation of multiple mKATE fluorescent foci. Upon florescence detection in greater than 70% of the monolayer, the virus was harvested. Mutations were confirmed by Sanger sequencing.
Viral stocks were titered by 50% tissue culture infectious dose (TCID50) in HEp-2 cells. The presence or absence of mKATE signal was assessed at 7 days postinfection. The Marco-Binder calculator was used to calculate TCID50/mL (Marco Binder, University of Heidelberg).
RNA Purification, cDNA Preparation, and PCR
Viral RNA was extracted from viral stocks and purified using the Quick-RNA Viral 96 Kit (Zymo Research). cDNA of the N ORF region of the RSV genome was created with SuperScript IV One-Step RT-PCR (Invitrogen) and Sanger sequenced using the primers listed in Supplementary Table 1.
In Vitro Compound Screen
HEp-2 cells were infected at a multiplicity of infection (MOI) of 0.1 and treated with the indicated compound or dimethyl sulfoxide (DMSO) at the time of infection, without removing the inoculum. mKATE fluorescent intensity was measured 5 days postinfection using a Molecular Device SpectraMax i3x. Percent viral inhibition was calculated by normalizing mKATE values to DMSO-treated infected cells. EC50/EC90 values were calculated using XLFit Dose-Response Model 200.
Viral Fitness Assays
HEp-2 cells were infected at an MOI of 0.1. mKATE fluorescent intensity was measured or cells and supernatant were collected for TCID50 analysis at each day postinfection for 7 days, as described above.
Statistical Analysis
All statistical analysis was performed using GraphPad Prism version 9.0 using analysis of variants (ANOVA) followed by a post hoc comparison test. All graphs show mean ± standard deviation. A minimum of 3 biological replicates were conducted for all dose-response and fitness experiments.
Next-Generation Sequencing
NGS methods are in Supplementary Methods.
RESULTS
Two Treatment-Emergent Mutations Were Observed in Samples from the Human Challenge Trial
To assess the emergence of resistance, viral loads from approximately 2480 nasal wash samples collected over 12 study days from the 124 subjects with RSV infection in the human challenge trial were evaluated by reverse transcription quantitative PCR (RT-qPCR) [11]. To maximize the likelihood of sequencing samples with EDP-938 resistance mutations, we employed a selective strategy for determining which participants and which participants’ samples to sequence. Samples were sequenced from patients treated with EDP-938 or placebo that had transient increases in viral load or a slow viral decline. The first category included EDP-938–treated participants with additional peaks of viral shedding ≥1 log from their previous nadir on or off therapy. These additional peaks of viral shedding could suggest the emergence of a variant with reduced sensitivity to EDP-938. This group was labeled group A and included 24 participants and 80 samples. The second group were EDP-938–treated participants that did not show a rapid or typical reduction in viral shedding compared to placebo-treated participants. The slower response could be indicative of the presence of a variant(s) with decreased sensitivity to EDP-938. This group was labeled group B and included 11 participants and 34 samples. The last group were placebo-treated participants, of whom some had an additional peak of viral shedding while most were randomly selected. These participants were included as controls to understand viral genotypic variance in the absence of EDP-938 selective pressure. These comprised group C and included 40 samples collected from 11 participants. Participants who had a response to EDP-938 with a consistent decline in viral load upon initiation of treatment followed by subsequently undetectable viral loads (group D) were judged to have the lowest probability of on-treatment detection of EDP-938–resistant variants, and thus were not selected for sequencing. Two participants (10035 and 20002) initially classified with additional peaks of shedding (group A) were reclassified into group D as there was a rapid decline in viral load following initiation of treatment. Nonetheless, 5 samples from these 2 participants underwent sequencing. The viral shedding patterns of all participants selected for sequencing are in Supplemental Figures 1–4. Theoretical viral load patterns that could be observed in each group are outlined in Figure 1B. Based on a percentage of the intention-to-treat infected population, the number of participants sequenced represents 45% of the EDP-938–treated participants and 26% of the placebo-treated participants. Group A compromised 65% of sequenced, EDP-938–treated participants, while groups B and D compromised 30% and 5%, respectively.
Figure 1.
Variants detected in the human challenge trial. A, Diagram detailing selection of participants for sequencing analysis. B, Theoretical viral load patterns for each group. These data are not from any participants but an example of viral load patterns that could be observed in each group. C and D, Viral shedding patterns for (C) LLOQ and (D) N:L139I for each participant from the human challenge trial with treatment-emergent mutations. Shaded triangles indicate EDP-938 dosing. Participant 20027 was assigned to a once daily treatment group. As such, this participant received a placebo for 1 of the 2 daily doses (open triangles). Light dashed line indicates the LLOQ (2.8 log10 copies/mL). Bold dashed line indicates the LLOD (1.4 log10 copies/mL). No mapped reads indicate no sequencing reads were able to be aligned to the RSV M37b reference genome. Abbreviations: LD, loading dose; LLOD, lower limit of detection; LLOQ, lower limit of quantification; N, nucleoprotein; RSV, respiratory syncytial virus; WT, wild type.
Specific nasal wash time point samples were chosen to enrich the likelihood of identifying variants with decreased sensitivity to EDP-938. When viral loads supported it, 3 time points were selected for sequencing: a pretreatment baseline, immediately prior to the triggering event (nadir), and immediately after the triggering event (postnadir). This enabled identification of variants and how they changed over the study. The target viral concentration for sequencing was 3.5 log10 copies/mL; however, in cases where samples did not meet the threshold, samples slightly below the cutoff were chosen. In total, 159 samples from 48 participants were chosen for sequencing (Figure 1A). Detailed information about samples is in Supplementary Table 2.
As in vitro resistance generation identified mutations in the N protein [9], we performed NGS on amplicons from the N region of the RSV genome. Samples had on average 87 000 reads after trimming and filtering. Detailed information about sequence reads and mapping is in Supplemental Tables 3–5. The input virus also underwent NGS as part of quality assurance mechanisms. As expected, the input virus matched the RSV-A M37b reference genome [21], and no variants were detected at the 1% level (accession number, KM360090). All sequences were aligned and compared with the RSV-A M37b reference genome. Four different SNPs were detected: 3 within the RSV N gene and 1 in the phosphoprotein (P) gene. Two SNPs (N:K5K, A1128G in RSV genome, and P:A5A, T2335A) were silent and present in most samples, including placebo-treated participants, at all time points (Supplementary Figure 5 and Supplementary Table 6). The other SNPs were changes in the N protein. N:E112G (A1448G) was detected in a single post–EDP-938 time point collected prior to the additional peak and was transient as all subsequent samples were WT until RSV was undetected (Figure 1C). N:L139I (C1528A) was detected in nasal wash samples collected after EDP-938 treatment on study day 6, Am and study day 7, Am in more than 50% of reads, indicating that it became the dominant variant (Figure 1D). After study day 7, Am, no further RSV was detected, indicating viral clearance (Figure 1D). Of the approximately 2480 samples from 124 participants with RSV infection in the study only 3 samples from 2 participants had treatment-emergent nonsynonymous mutations. Neither treatment-emergent mutation impaired viral clearance. Importantly, neither N:E112G nor N:L139I were detected in any pretreatment samples.
N:L139I But Not N:E112G Reduces the Sensitivity of RSV to EDP-938
To determine how N:E112G or N:L139I affect the sensitivity of RSV to EDP-938, we created recombinant RSV strains with these mutations [20]. The EC50/90 of EDP-938 against N:E112G was comparable to those values obtained against a recombinant WT virus (Table 1 and Figure 2A). However, N:L139I resulted in an approximately 10-fold decrease in both the EC50 and EC90 of EDP-938 (Table 1 and Figure 2A).
Table 1.
Antiviral Activity of Anti-RSV Compounds Against Identified Variants
| Compound | WT | N:L139I | N:E112G | |||
|---|---|---|---|---|---|---|
| EC50, nM | EC90, nM | EC50, nM | EC90, nM | EC50, nM | EC90, nM | |
| EDP-938 | 61 ± 12 | 91 ± 5 | 590 ± 69 | 1075 ± 388 | 100 ± 33 | 124 ± 34 |
| EDP-323 | 0.21 ± 0.04 | 0.37 ± 0.08 | 0.14 ± 0.04 | 0.31 ± 0.03 | 0.10 ± 0.04 | 0.14 ± 0.03 |
| AK0529 | 7 ± 1 | 25 ± 18 | 9 ± 6 | 52 ± 41 | 2 ± 1 | 15 ± 8 |
| RV521 | 0.42 ± 0.32 | 2.69 ± 2.84 | 0.37 ± 0.11 | 1.24 ± 0.80 | 0.62 ± 0.38 | 3.97 ± 5.02 |
For each biological replicate, a 4-parameter logistic regression using the 200 dose-response model in XLFit was applied to the curves in Figure 2 and the EC50 and EC90 are reported as the mean ± standard deviation from at least 3 independent biological replicates.
Abbreviations: EC50, 50% maximum effective concentration; EC90, 90% maximum effective concentration; WT, wild type.
Figure 2.
N:L139I mediates resistance to EDP-938. HEp-2 cells were infected with the indicated recombinant strain of RSV-A expressing an mKATE reporter at a multiplicity of infection of 0.1. At the time of infection, cells were treated with the indicated concentration of the listed compound or DMSO vehicle control: (A) EDP-938, (B) EDP-323, (C) AK0529, and (D) RV521. Five days postinfection, the mKATE fluorescence intensity was measured. Percent viral inhibition at each concentration was calculated. Data are expressed as the mean percent viral inhibition at each concentration ± standard deviation from at least 3 independent biological replicates. Dotted lines indicate 50% and 90% viral inhibition. Abbreviations: DMSO, dimethyl sulfoxide; N, nucleoprotein; RSV, respiratory syncytial virus; WT, wild type.
We tested these recombinant viruses against AK0529, RV521, and EDP-323. AK0529 is a fusion inhibitor that completed a phase 3 trial in China (AirFLO, NCT04231968), and reduced symptoms and viral loads [22]. RV521 is also a fusion inhibitor that reduced symptom scores and viral loads in a human challenge trial [16]. EDP-323 is a nonnucleoside large (L)-polymerase inhibitor with picomolar in vitro potency and in vivo efficacy in a mouse model of RSV infection [23, 24]. EDP-323 completed phase 1 clinical trials and was generally safe and well tolerated in healthy volunteers [25]. None of the variants decreased the sensitivity of the virus to any of these compounds (Table 1 and Figure 2B–D).
N:L139I Reduces In Vitro Fitness of RSV
To determine the effects of N:E112G or N:L139I on viral fitness, we analyzed in vitro growth kinetics. As the recombinant viruses express mKATE, we measured the mKATE florescent signal each day following infection for 7 days. Comparing the area under the curve (AUC) of the mKATE signal over 7 days showed that signals were comparable to WT for all mutant viruses (Figure 3A and 3B). While mKATE signal measures the production of viral mRNA and its subsequent translation, it does not necessarily translate to infectious virus production. Thus, we measured infectious virion production over 7 days. N:L139I took 7 days to achieve its peak viral titer while WT achieved peak titer in 6 days, suggesting a slight in vitro growth delay. The peak titer for N:E112G was obtained 1 day before that of WT. The AUC of the infectious virus produced over 7 days for N:L139I was only approximately 50% of that of the WT (Figure 3C and 3D), further suggesting reduced viral fitness for N:L139I. No significant changes in AUC were observed for N:E112G (Figure 3C and 3D). These data demonstrate that resistance to EDP-938 is associated with reduced viral fitness.
Figure 3.
N:L139I has in vitro fitness defects compared to WT. A and C, HEp-2 cells were infected with the indicated recombinant strain of RSV-A expressing an mKATE reporter at a multiplicity of infection of 0.1. At each day postinfection, mKATE florescence intensity was measured (A) or cells and supernatant were harvested and subject to TCID50 analysis (C). Data are expressed as the mean mKATE florescence intensity (A) or TCID50/mL (C) at each day postinfection ± standard deviation from at least 3 independent biological replicates. LoD was 3.16E + 0 TCID50/mL. Values at or below the LoD are set at 2.0E + 0 TCID50/mL. B and D, For each replicate, the AUC was determined using a linear-linear plot. The AUC was normalized to WT and expressed as the percent AUC relative to WT. Data are expressed as the mean ± standard deviation AUC. *P < .05 by 1-way repeated measured ANOVA followed by Dunnett multiple comparison test. Abbreviations: AUC, area under the curve; LOD, limit of detection; N, nucleoprotein; ns, not significant; RSV, respiratory syncytial virus; TCID50, 50% tissue culture infectious dose; WT, wild type.
DISCUSSION
Like all antimicrobials, generation of antiviral resistance is a major concern. We report that only 1 EDP-938–treated participant out of the 37 sequenced (approximately 3%) had a treatment-emergent mutation associated with resistance (Figure 1, Figure 2, and Table 1). Additionally, in vitro characterization of mutant viruses suggests that resistance is associated with reduced fitness (Figure 2 and Figure 3). Moreover, clinical resistance to EDP-938 was not associated with resistance to other classes of inhibitors (Figure 2). Lastly, resistance to EDP-938 did not impair viral clearance, as all remaining virus was cleared shortly after detection of N:L139I (Figure 1).
We identified a single treatment-emergent resistant mutant, N:L139I, which was also identified in preclinical resistance studies of RSV-604 [26], another RSV N inhibitor, whose clinical development was discontinued [27]. N:L139I resulted in an approximately 7-fold decrease in in vitro RSV-604 potency [26]. Similar results were obtained for EDP-938 (Table 1). N:L139I was not detected in EDP-938 preclinical resistance generation. Nonetheless, N:L139I confers reduced viral fitness as it reduced both the AUC of infectious virus and increased the time to peak titer (Figure 3). While there was no significant change in mKATE intensity (Figure 3), this metric is not an indication of infectious virus. In contrast, this variant was rapidly cleared in the clinical setting (Figure 1) in an otherwise healthy participant that can mount an effective immune response, further suggesting reduced viral fitness. Nonetheless, the isolation of N:L139I in 2 independent N-inhibitor studies suggests the need for continued vigilance in clinical development.
There appeared to be a complete loss of viral shedding in participant 20027 between the 2 detections of N:L139I (Figure 1). The lack of detection could be due to sampling variation, the dilution of the sample associated with nasal washing, and/or remnants of noninfectious viral RNA released from dead or dying infected cells. However, it is also possible that infectious virus below the limit of detection at study day 6, Pm was rapidly replicated to near baseline levels by study day 7, Am. The possibility of a multi-log increase is unlikely, especially given N:L139I's reduced viral fitness (Figure 3). The percentage of reads that were N:L139I only changed approximately 10% from study day 6, Am compared study day 7, Am (Figure 1) with less than a log change in viral load, suggesting there was not infectious virus below the limit of detection on study day 6, Pm. Most of the groups A and D show similar patterns to participant 20027, that is a time point with lack of detection sandwiched between 2 time points with similar viral loads (Supplemental Figures 1–4). The additional peak could be the result of sampling variation, especially when viral loads are just barely above the limit of detection/quantification and/or release of noninfectious viral RNA from dead or dying cells. Given that the goal with participant selection for sequencing was to maximize the detection of resistance, we likely overclassified participants into groups associated with additional peaks of shedding or a reduced response to not miss variants. Nonetheless, this overclassification also emphasizes the relatively low occurrence of treatment-emergent mutations when compared to Presatovir, where more than 20% of participants had treatment-emergent mutations in a human challenge trial [15].
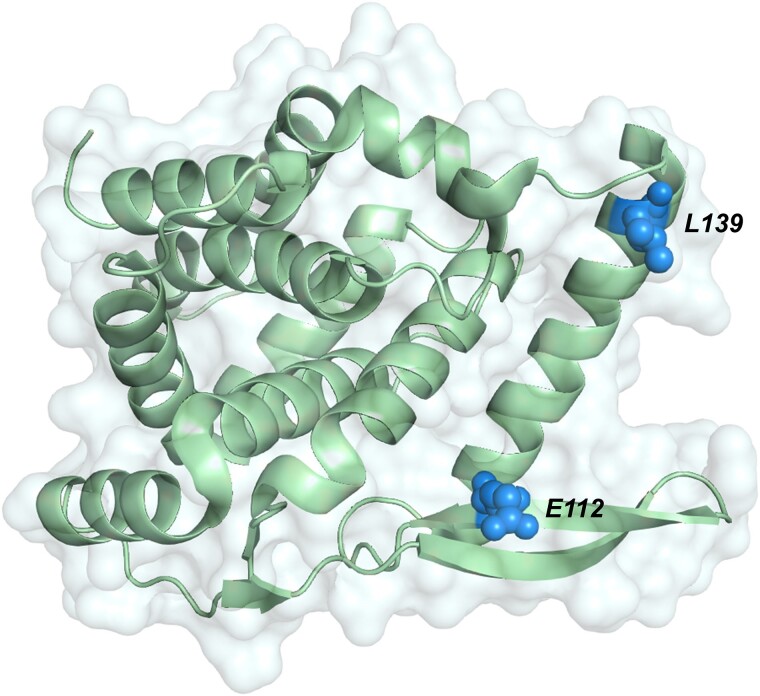
The 2 observed nonsynonymous mutations are located near where the P proteins are hypothesized to interact with N [9, 28, 29] (Figure 4, right). In EDP-938 in vitro resistance generation studies, most of the identified mutations were also in this area, although many did not mediate meaningful changes in antiviral potency [9]. It is unlikely that EDP-938 disrupts N-P interactions, as EDP-938 has no effect on either mRNA transcription or genome replication in a minigenome system [9] and the similar RSV inhibitor, RSV-604, had minimal effect in a replicon assay [30]. It is currently hypothesized that EDP-938 exerts its antiviral activity either directly on the N protein or through a cellular protein [9]. Further elucidation of the mechanism of action of EDP-938 will enable determination of the role of these residues, especially with respect to N:L139I.
Figure 4.
Mapping of observed mutations on the nucleoprotein (N) crystal structure. N crystal structure [9] (Protein Date Bank file No. 4UC6) of amino acids 32–251 containing all observed mutations in this region. Observed mutated residues are shown as spheres.
Our results highlight EDP-938's higher barrier to resistance relative to fusion inhibitors, differentiating EDP-938 from other small-molecule RSV therapies in clinical development. In a clinical trial setting, development of resistance to fusion inhibitors is well documented. In a human challenge trial, approximately 28% of Presatovir-treated participants had treatment-emergent mutations, with 17% of Presatovir-treated participants having resistance-associated amino acids [15]. We did not sequence every participant and are unable to determine the exact percentage of participants with treatment-emergent mutations. However, in a population preselected to have higher likelihood of resistance mutations, only 2 of 37 or 5% of EDP-938–treated participants had treatment-emergent mutations and only 1 (approximately 3%) reduced the sensitivity of the virus to EDP-938, which is less than one-fifth the number of Presatovir-treated participants. Another key difference is that Presatovir-resistant variants were associated with higher viral load AUCs versus nonresistant variants [15]. These data are in direct contrast to EDP-938, where the resistant variant was rapidly cleared (Figure 1). Together these data highlight a key advantage of EDP-938 over fusion inhibitors.
EDP-938's improved barrier to resistance relative to that of fusion inhibitors may be explained by its mechanism of action. EDP-938 targets the nucleoprotein, the most conserved viral protein [31]. Mutations that drastically decrease the susceptibility of the virus to EDP-938 may not be tolerated as they could disrupt an essential function(s) of N. The low mutability of N may account for EDP-938's observed high barrier to resistance, which is in direct contrast to fusion protein targeted therapies. The fusion protein is mutable and associated with sequence variability within the antigenic sites [32], suggesting an ability to adapt to antibody responses, including palivizumab. Palivizumab-resistant mutants have been recovered from 5%–10% of children hospitalized with RSV breakthrough infections [33–37]. The same adaptability likely applies to DAAs that target the F protein, including Presatovir.
Collectively, our results demonstrate EDP-938's high barrier to resistance in a clinical setting, differentiating EDP-938 from other RSV DAAs. It will be critical to evaluate resistance development in the background of pediatric and immunocompromised patients, who have longer durations of viral shedding and may not be able to adequately mount of a complete immune response [38].
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Rachel Emily Levene, Enanta Pharmaceuticals, Inc, Watertown, Massachusetts, USA.
John DeVincenzo, Enanta Pharmaceuticals, Inc, Watertown, Massachusetts, USA.
Annie L Conery, Enanta Pharmaceuticals, Inc, Watertown, Massachusetts, USA.
Alaa Ahmad, Enanta Pharmaceuticals, Inc, Watertown, Massachusetts, USA.
Yat Sun Or, Enanta Pharmaceuticals, Inc, Watertown, Massachusetts, USA.
Michael H J Rhodin, Enanta Pharmaceuticals, Inc, Watertown, Massachusetts, USA.
Notes
Author contributions . R. E. L., J. D., A. L. C., A. A., Y. S. O., and M. H. J. R. contributed study conception. R. E. L. and hVIVO collected data. R. E. L., J. D., A. L. C., A. A., and M. H. J. R. analyzed data. J. D., A. L. C., A. A., Y. S. O., and M. H. J. R. contributed supervision. R. E. L. wrote the manuscript. All authors reviewed the manuscript.
Acknowledgments. We extend a special thank you to the participants in this clinical trial, their families, and study site investigators. We thank members of the biology, DMPK, toxicology, medicinal chemistry, and clinical/regulatory teams at Enanta Pharmaceuticals for suggestions, feedback, critical reading of the manuscript, and development of EDP-938. We also thank the team at hVIVO for conducting the human challenge trial and sequencing of the samples from human challenge trial participants.
Financial support . This work was supported by Enanta Pharmaceuticals, Inc.
Data availability. Data available upon request.
References
- 1. Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica 2019; 104:1322–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 2005; 352:1749–59. [DOI] [PubMed] [Google Scholar]
- 3. Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009; 360:588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shook BC, Lin K. Recent advances in developing antiviral therapies for respiratory syncytial virus. Top Curr Chem 2017; 375:40. [DOI] [PubMed] [Google Scholar]
- 5. Meissner HC, Rennels MB, Pickering LK, Hall CB. Risk of severe respiratory syncytial virus disease, identification of high risk infants and recommendations for prophylaxis with palivizumab. Pediatr Infect Dis J 2004; 23:284–5. [DOI] [PubMed] [Google Scholar]
- 6. Food and Drug Administration . FDA approves new drug to prevent RSV in babies and toddlers, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-prevent-rsv-babies-and-toddlers. Accessed 4 August 2023.
- 7.Centers for Disease Control and Prevention. RSV vaccine guidance for older adults. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults-faqs.html. Accessed 18 December 2023.
- 8. Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhodin MHJ, McAllister NV, Castillo J, et al. EDP-938, a novel nucleoprotein inhibitor of respiratory syncytial virus, demonstrates potent antiviral activities in vitro and in a non-human primate model. PLoS Pathog 2021; 17:e1009428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adda N, Dickerson D, Sanderson K, Ahmad A. EDP-938, a novel, non-fusion replication inhibitor of respiratory syncytial virus: preliminary results of a phase 1 study in healthy subjects (HS). 11th International Respiratory Syncytial Virus Symposium, Asheville, NC, 31 October-4 November 2018, poster 104. [Google Scholar]
- 11. Ahmad A, Eze K, Noulin N, et al. EDP-938, a respiratory syncytial virus inhibitor, in a human virus challenge. N Engl J Med 2022; 386:655–66. [DOI] [PubMed] [Google Scholar]
- 12. Perron M, Stray K, Kinkade A, et al. GS-5806 inhibits a broad range of respiratory syncytial virus clinical isolates by blocking the virus-cell fusion process. Antimicrob Agents Chemother 2015; 60:1264–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng X, Gao L, Wang L, et al. Discovery of Ziresovir as a potent, selective, and orally bioavailable respiratory syncytial virus fusion protein inhibitor. J Med Chem 2019; 62:6003–14. [DOI] [PubMed] [Google Scholar]
- 14. Cockerill GS, Angell RM, Bedernjak A, et al. Discovery of Sisunatovir (RV521), an inhibitor of respiratory syncytial virus fusion. J Med Chem 2021; 64:3658–76. [DOI] [PubMed] [Google Scholar]
- 15. Stray K, Perron M, Porter DP, et al. Drug resistance assessment following administration of respiratory syncytial virus (RSV) fusion inhibitor Presatovir to participants experimentally infected with RSV. J Infect Dis 2020; 222:1468–77. [DOI] [PubMed] [Google Scholar]
- 16. DeVincenzo J, Tait D, Efthimiou J, et al. A randomized, placebo-controlled, respiratory syncytial virus human challenge study of the antiviral efficacy, safety, and pharmacokinetics of RV521, an inhibitor of the RSV-F protein. Antimicrob Agents Chemother 2020; 64:e01884–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Porter DP, Guo Y, Perry J, et al. Assessment of drug resistance during phase 2b clinical trials of Presatovir in adults naturally infected with respiratory syncytial virus. Antimicrob Agents Chemother 2020; 64:e02312–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Battles MB, Langedijk JP, Furmanova-Hollenstein P, et al. Molecular mechanism of respiratory syncytial virus fusion inhibitors. Nat Chem Biol 2016; 12:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang W, Li Y, Song Q, et al. Mechanism of cross-resistance to fusion inhibitors conferred by the K394R mutation in respiratory syncytial virus fusion protein. J Virol 2021; 95:e0120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hotard AL, Shaikh FY, Lee S, et al. A stabilized respiratory syncytial virus reverse genetics system amenable to recombination-mediated mutagenesis. Virology 2012; 434:129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim Y-I, DeVincenzo JP, Jones BG, et al. Respiratory syncytial virus human experimental infection model: provenance, production, and sequence of low-passaged Memphis-37 challenge virus. PLoS One 2014; 9:e113100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ArkBio . Ark Biopharmaceutical announces positive results of phase 3 study with Ziresovir in infants and children hospitalized with respiratory syncytial virus infection. https://www.arkbiosciences.com/en_2022n/110. Accessed 14 December 2022.
- 23. Rhodin MHJ. EDP-323, a novel L-protein inhibitor, for the treatment of respiratory syncytial virus. Belfast, Northern Ireland, UK, 29 September–2 October, 2022. [Google Scholar]
- 24. Levene RE. In vivo efficacy of EDP-323, a novel L-protein inhibitor, for the treatment of respiratory syncytial virus. Belfast, Northern Ireland, UK, 29 September–2 October, 2023. [Google Scholar]
- 25. Enanta Pharmaceuticals, Inc . Enanta Pharmaceuticals announces positive data from a phase 1 clinical study of EDP-323, an oral, L-protein inhibitor in development for the treatment of respiratory syncytial virus. https://ir.enanta.com/news-releases/news-release-details/enanta-pharmaceuticals-announces-positive-data-phase-1-0/. Accessed 4 August 2023.
- 26. Chapman J, Abbott E, Alber DG, et al. RSV604, a novel inhibitor of respiratory syncytial virus replication. Antimicrob Agents Chemother 2007; 51:3346–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chapman J, Cockerill G. Discovery and development of RSV604. In: Kazmieski WM, ed. Antiviral drugs: from basic discovery through clinical trials. Hoboken, NJ: John Wiley and Sons, 2011, 367–84 [Google Scholar]
- 28. Ouizougun-Oubari M, Pereira N, Tarus B, et al. A druggable pocket at the nucleocapsid/phosphoprotein interaction site of human respiratory syncytial virus. J Virol 2015; 89:11129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galloux M, Tarus B, Blazevic I, Fix J, Duquerroy S, Eléouët J-F. Characterization of a viral phosphoprotein binding site on the surface of the respiratory syncytial nucleoprotein. J Virol 2012; 86:8375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Challa S, Scott AD, Yuzhakov O, et al. Mechanism of action for respiratory syncytial virus inhibitor RSV604. Antimicrob Agents Chemother 2015; 59:1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Collins PL, Fearns R, Graham BS. Respiratory syncytial virus: virology, reverse genetics, and pathogenesis of disease. Curr Top Microbiol Immunol 2013; 372:3–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mas V, Nair H, Campbell H, Melero JA, Williams TC. Antigenic and sequence variability of the human respiratory syncytial virus F glycoprotein compared to related viruses in a comprehensive dataset. Vaccine 2018; 36:6660–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. DeVincenzo JP, Hall CB, Kimberlin DW, et al. Surveillance of clinical isolates of respiratory syncytial virus for palivizumab (Synagis)-resistant mutants. J Infect Dis 2004; 190:975–8. [DOI] [PubMed] [Google Scholar]
- 34. Adams O, Bonzel L, Kovacevic A, Mayatepek E, Hoehn T, Vogel M. Palivizumab-resistant human respiratory syncytial virus infection in infancy. Clin Infect Dis 2010; 51:185–8. [DOI] [PubMed] [Google Scholar]
- 35. Papenburg J, Carbonneau J, Hamelin M-È, et al. Molecular evolution of respiratory syncytial virus fusion gene, Canada, 2006–2010. Emerg Infect Dis 2012; 18:120–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu Q, McAuliffe JM, Patel NK, et al. Analysis of respiratory syncytial virus preclinical and clinical variants resistant to neutralization by monoclonal antibodies palivizumab and/or motavizumab. J Infect Dis 2011; 203:674–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oliveira DBL, Iwane MK, Prill MM, et al. Molecular characterization of respiratory syncytial viruses infecting children reported to have received palivizumab immunoprophylaxis. J Clin Virol 2015; 65:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr 2000; 137:78–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.