Abstract
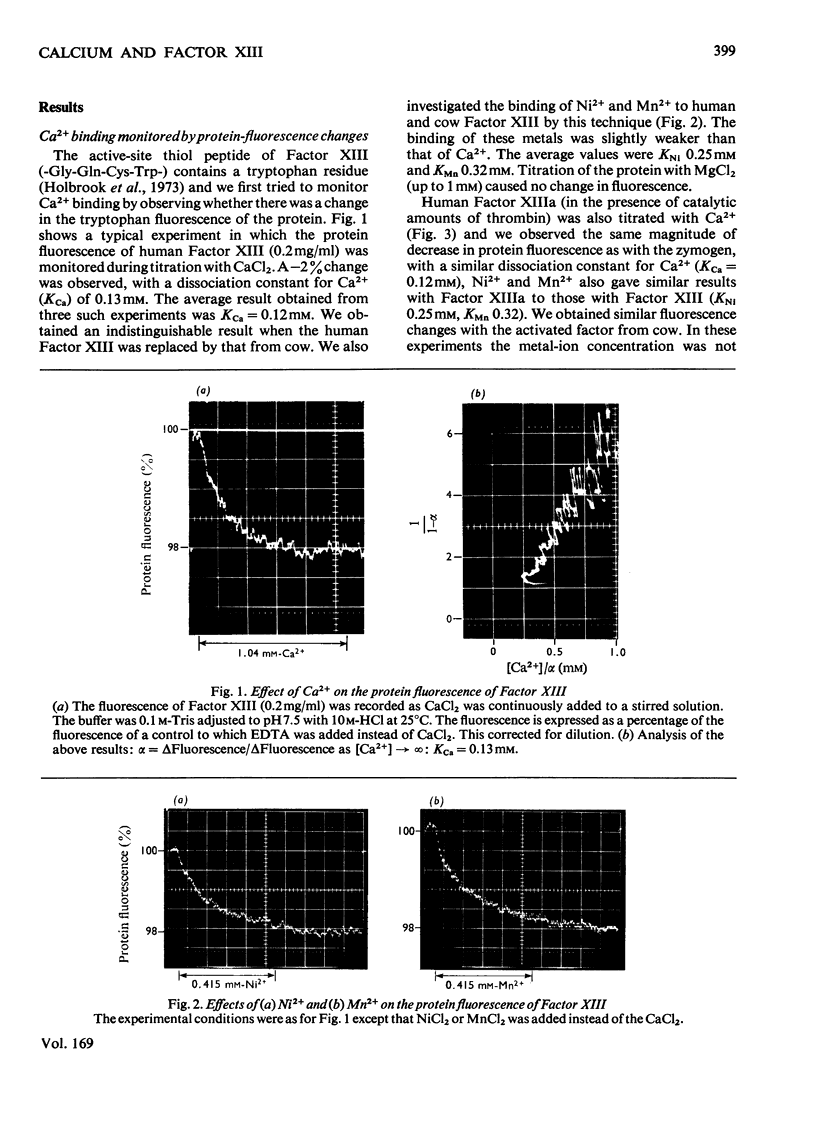
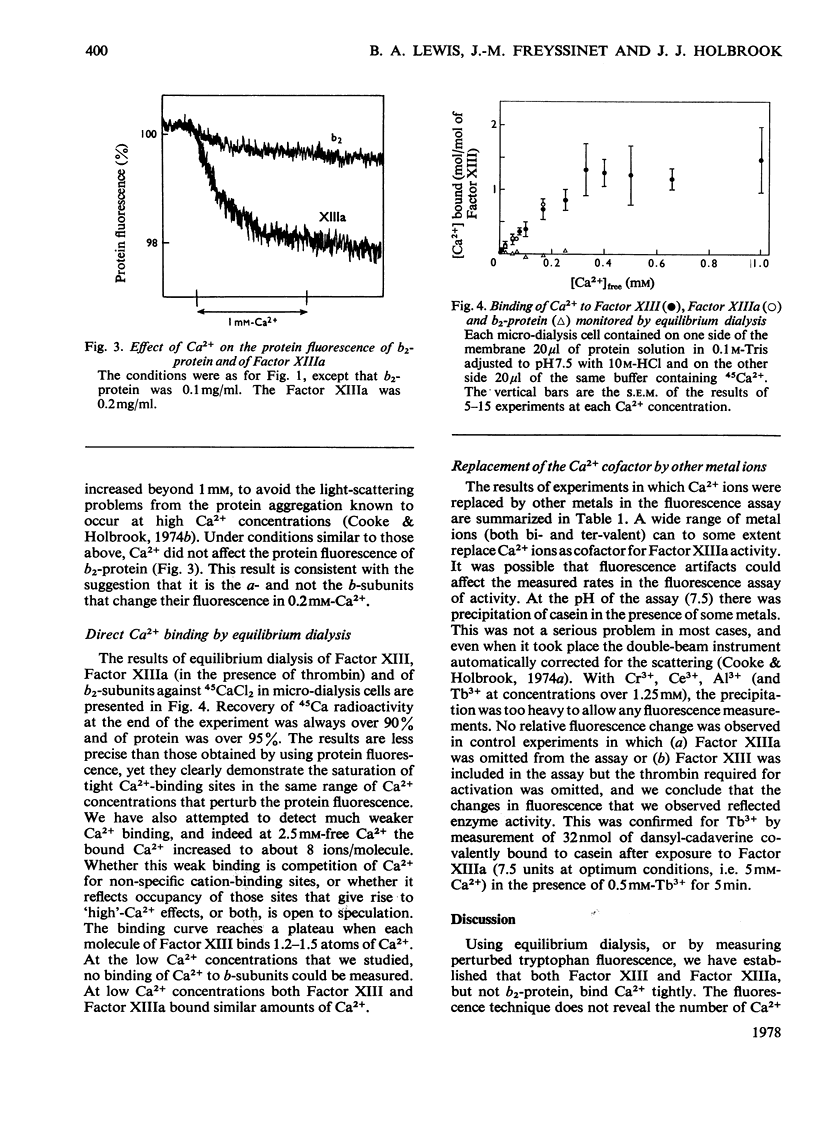
1. The binding of Ca2+ to plasma coagulation Factor XIII from man and from cow caused a small decrease in the intrinsic fluorescence of the protein with a dissociation constant of 0.1 mM. A similar decrease was observed with the thrombin-activated Factors (Factors XIIa). The decrease in protein fluorescence was also caused by both Ni2+ and Mn2+ but not by Mg2+. 2. 45Ca2+ binding was directly demonstrated by equilibrium dialysis. Ca2+ at 0.2 mM bound to Factor XIII (a2b2) and Factor XIIIa (a'2b2) but not to isolated b2-protein. A tight-binding site for Ca2+ is associated with the a-subunits. 3. The Ca2+ essential for the enzyme activity of Factor XIII from man, pig and cow can be replaced by Ni2+, Cu2+, La3+, Mn2+, Fe3+, Y3+, Co2+, Sr2+ or Tb3+, but not by Mg2+.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asquith R. S., Otterburn M. S., Buchanan J. H., Cole M., Fletcher J. C., Gardner K. L. The identification of epsilon-N-(gamma-L-glutamyl)-L-lysine cross-links in native wool keratins. Biochim Biophys Acta. 1970 Nov 17;221(2):342–348. doi: 10.1016/0005-2795(70)90274-6. [DOI] [PubMed] [Google Scholar]
- Buxman M. M., Wuepper K. D. Isolation, purification and characterization of bovine epidermal transglutaminase. Biochim Biophys Acta. 1976 Dec 8;452(2):356–369. doi: 10.1016/0005-2744(76)90185-6. [DOI] [PubMed] [Google Scholar]
- Chung S. I., Folk J. E. Kinetic studies with transglutaminases. The human blood enzymes (activated coagulation factor 13 and the guinea pig hair follicle enzyme. J Biol Chem. 1972 May 10;247(9):2798–2807. [PubMed] [Google Scholar]
- Cooke R. D. Calcium-induced dissociation of human plasma factor XIII and the appearance of catalytic activity. Biochem J. 1974 Sep;141(3):683–691. doi: 10.1042/bj1410683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. Calcium and the assays of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):71–78. doi: 10.1042/bj1410071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Holbrook J. J. The calcium-induced dissociation of human plasma clotting factor XIII. Biochem J. 1974 Jul;141(1):79–84. doi: 10.1042/bj1410079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. D., Pestell T. C., Holbrook J. J. Calcium and thiol reactivity of human plasma clotting factor XIII. Biochem J. 1974 Sep;141(3):675–682. doi: 10.1042/bj1410675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis C. G., Brown K. L., Credo R. B., Domanik R. A., Gray A., Stenberg P., Lorand L. Calcium-dependent unmasking of active center cysteine during activation of fibrin stabilizing factor. Biochemistry. 1974 Aug 27;13(18):3774–3780. doi: 10.1021/bi00715a024. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1–109. doi: 10.1016/s0065-3233(08)60446-5. [DOI] [PubMed] [Google Scholar]
- Esnouf M. P., Lloyd P. H., Jesty J. A method for the simultaneous isolation of factor X and prothrombin from bovine plasma. Biochem J. 1973 Apr;131(4):781–789. doi: 10.1042/bj1310781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Harding H. W., Rogers G. E. Formation of the -( -glutamyl) lysine cross-link in hair proteins. Investigation of transamidases in hair follicles. Biochemistry. 1972 Jul 18;11(15):2858–2863. doi: 10.1021/bi00765a019. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Cooke R. D., Kingston I. B. The amino acid sequence around the reactive cysteine residue in human plasma Factor XII. Biochem J. 1973 Dec;135(4):901–903. doi: 10.1042/bj1350901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keski-Oja J., Mosher D. F., Vaheri A. Cross-linking of a major fibroblast surface-associated glycoprotein (fibronectin) catalyzed by blood coagulation factor XIII. Cell. 1976 Sep;9(1):29–35. doi: 10.1016/0092-8674(76)90049-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Schwartz M. L., Pizzo S. V., Hill R. L., McKee P. A. Human Factor XIII from plasma and platelets. Molecular weights, subunit structures, proteolytic activation, and cross-linking of fibrinogen and fibrin. J Biol Chem. 1973 Feb 25;248(4):1395–1407. [PubMed] [Google Scholar]
- Stenberg P., Curtis C. G., Wing D., Tong Y. S., Credo R. B., Gray A., Lorand L. Transamidase kinetics. Amide formation in the enzymic reactions of thiol esters with amines. Biochem J. 1975 Apr;147(1):155–163. [PMC free article] [PubMed] [Google Scholar]
- Takagi T., Konishi K. Purification and some properties of fibrin stabilizing factor. Biochim Biophys Acta. 1972 Jul 21;271(2):363–370. doi: 10.1016/0005-2795(72)90211-5. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Cooper R. H., Denton R. M., Bridges B. J., Randle P. J. The elementary reactions of the pig heart pyruvate dehydrogenase complex. A study of the inhibition by phosphorylation. Biochem J. 1976 Jul 1;157(1):41–67. doi: 10.1042/bj1570041. [DOI] [PMC free article] [PubMed] [Google Scholar]




