Abstract
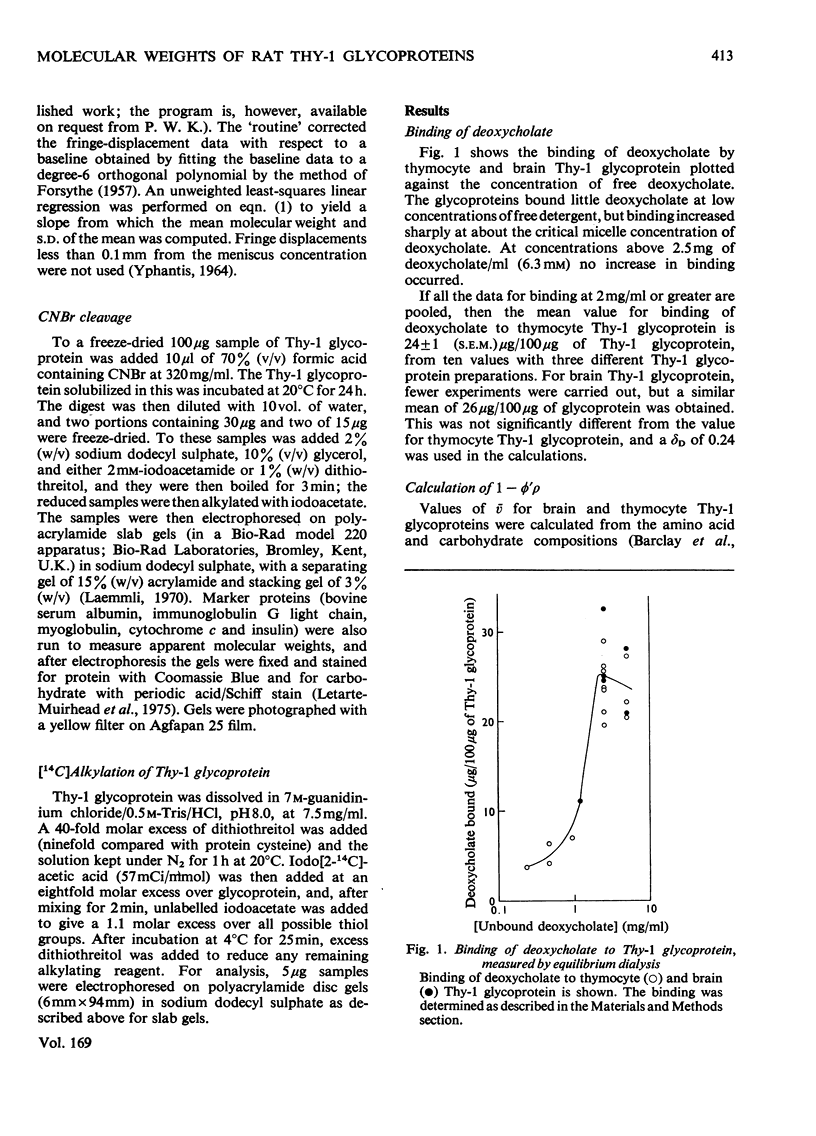
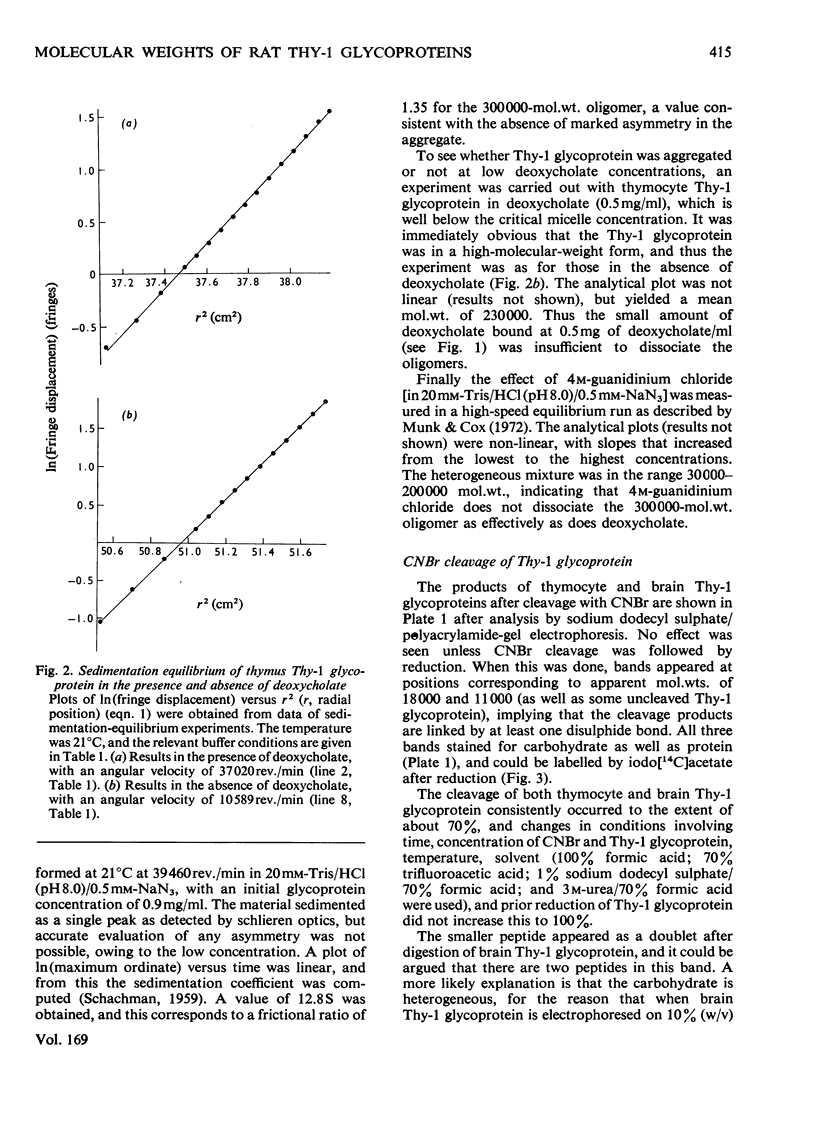
1. The Thy-1 membrane glycoproteins from rat thymus and brain bound deoxycholate to 24% of their own weight as measured by equilibrium dialysis. The binding occurred co-operatively at the critical micelle concentration of deoxycholate, suggesting that the glycoproteins bind to a micelle, and not to the detergent monomer. 2. From sedimentation-equilibrium and deoxycholate-binding data the molecular weights of the glycoprotein monomers were calculated to be 18700 and 17500 for thymus and brain Thy-1 glycoprotein monomers were calculated to be 18700 and 17500 for thymus and brain Thy-1 glycoproteins respectively. The molecular weight of the polypeptide part of the glycoprotein is thus 12500. 3. In the absence of deoxycholate, brain or thymus Thy-1 glycoprotein formed large homogeneous complexes of mol. wt. 270000 or 300000 respectively. The sedimentation coefficient of these was 12.8 S. The complex was only partially dissociated by 4M-guanidinium chloride. 4. After cleavage of brain or thymus Thy-1 glycoprotein with CNBr, two peptides were clearly identified. They were linked by disulphide bonds and both contained carbohydrate. This cleavage suggests there is only one methionine residue per molecule, which is consistent with the above molecular weights and the known amino acid composition.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F. Purification of the Thy-1 molecule from rat brain. Biochem J. 1975 Dec;151(3):699–706. doi: 10.1042/bj1510699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Freed J. H., Mole L. E., Nathenson S. G. Localization of the papain cleavage site of H-2 glycoproteins. Proc Natl Acad Sci U S A. 1976 Mar;73(3):915–918. doi: 10.1073/pnas.73.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letarte-Muirhead M., Barclay A. N., Williams A. F. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975 Dec;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muirhead M. L., Action R. T., Williams A. F. Preliminary characterization of Thy-1.1 and Ag-B antigens from rat tissues solubilized in detergents. Biochem J. 1974 Oct;143(1):51–61. doi: 10.1042/bj1430051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munk P., Cox D. J. Sedimentation equilibrium of protein solutions in concentrated guanidinium chloride. Thermodynamic nonideality and protein heterogeneity. Biochemistry. 1972 Feb 29;11(5):687–697. doi: 10.1021/bi00755a004. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Tanford C. Determination of molecular weight of the protein moiety in protein-detergent complexes without direct knowledge of detergent binding. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4467–4470. doi: 10.1073/pnas.73.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson N. C., Tanford C. The binding of deoxycholate, Triton X-100, sodium dodecyl sulfate, and phosphatidylcholine vesicles to cytochrome b5. Biochemistry. 1975 Jan 28;14(2):369–378. doi: 10.1021/bi00673a025. [DOI] [PubMed] [Google Scholar]
- Spatz L., Strittmatter P. A form of cytochrome b5 that contains an additional hydrophobic sequence of 40 amino acid residues. Proc Natl Acad Sci U S A. 1971 May;68(5):1042–1046. doi: 10.1073/pnas.68.5.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Teller D. C. Characterization of proteins by sedimentation equilibrium in the analytical ultracentrifuge. Methods Enzymol. 1973;27:346–441. doi: 10.1016/s0076-6879(73)27017-9. [DOI] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Weissman I. L., Bevan M. J. Mouse T-cell surface glycoprotein recognised by heterologous anti-thymocyte sera and its relationship to Thy-1 antigen. Nature. 1975 Aug 21;256(5519):652–654. doi: 10.1038/256652a0. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Letarte-Muirhead M., Morris R. J. Rat thy-1 antigens from thymus and brain: their tissue distribution, purification, and chemical composition. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):51–61. doi: 10.1101/sqb.1977.041.01.009. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



