Abstract
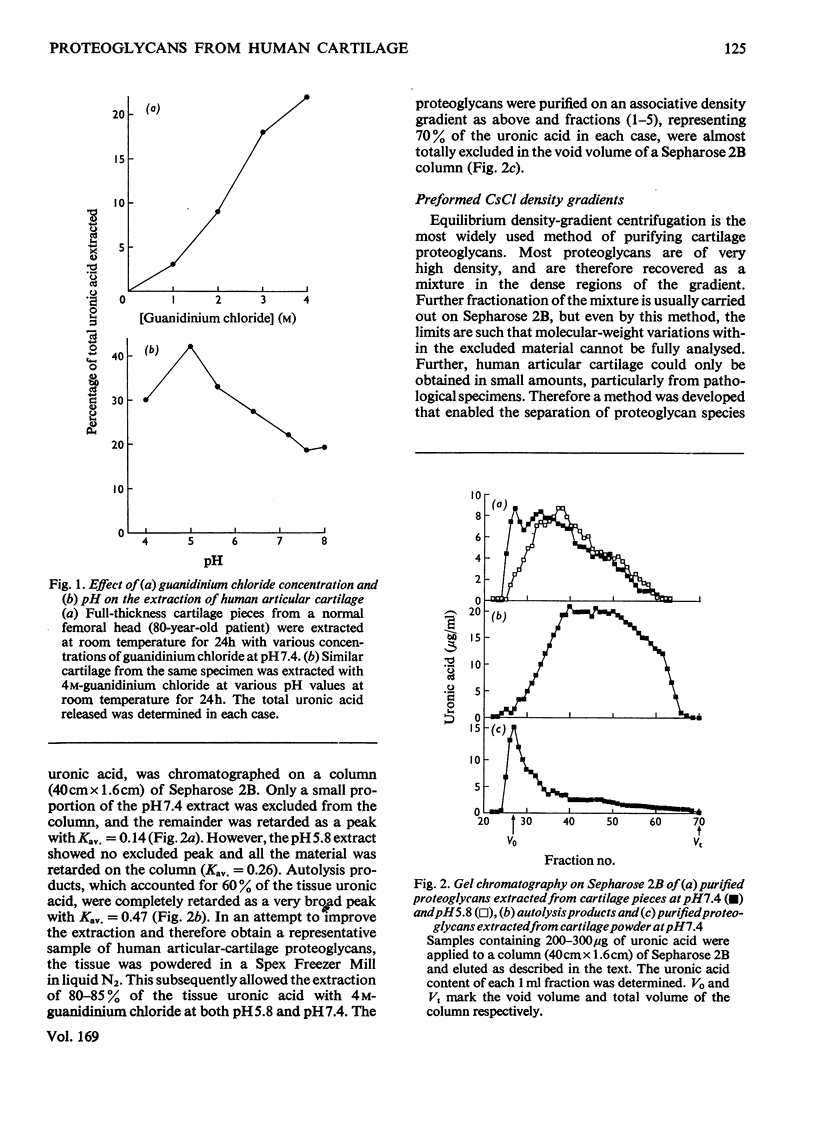
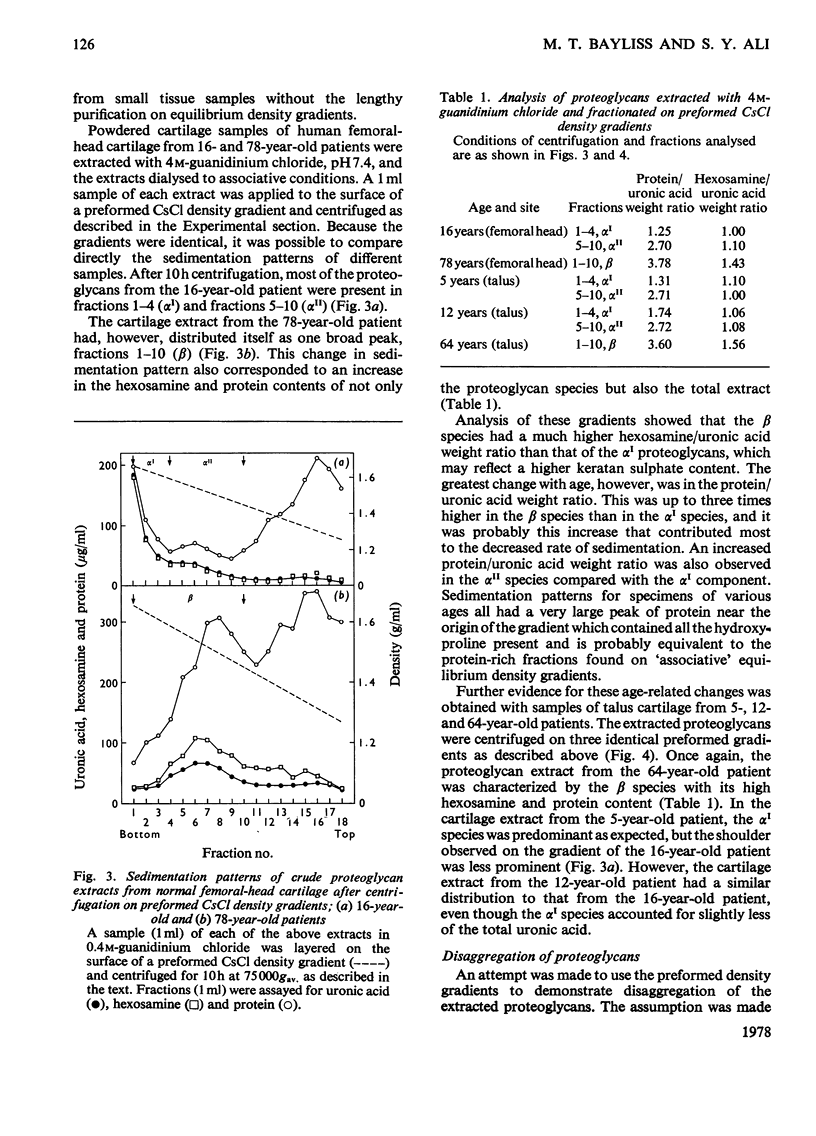
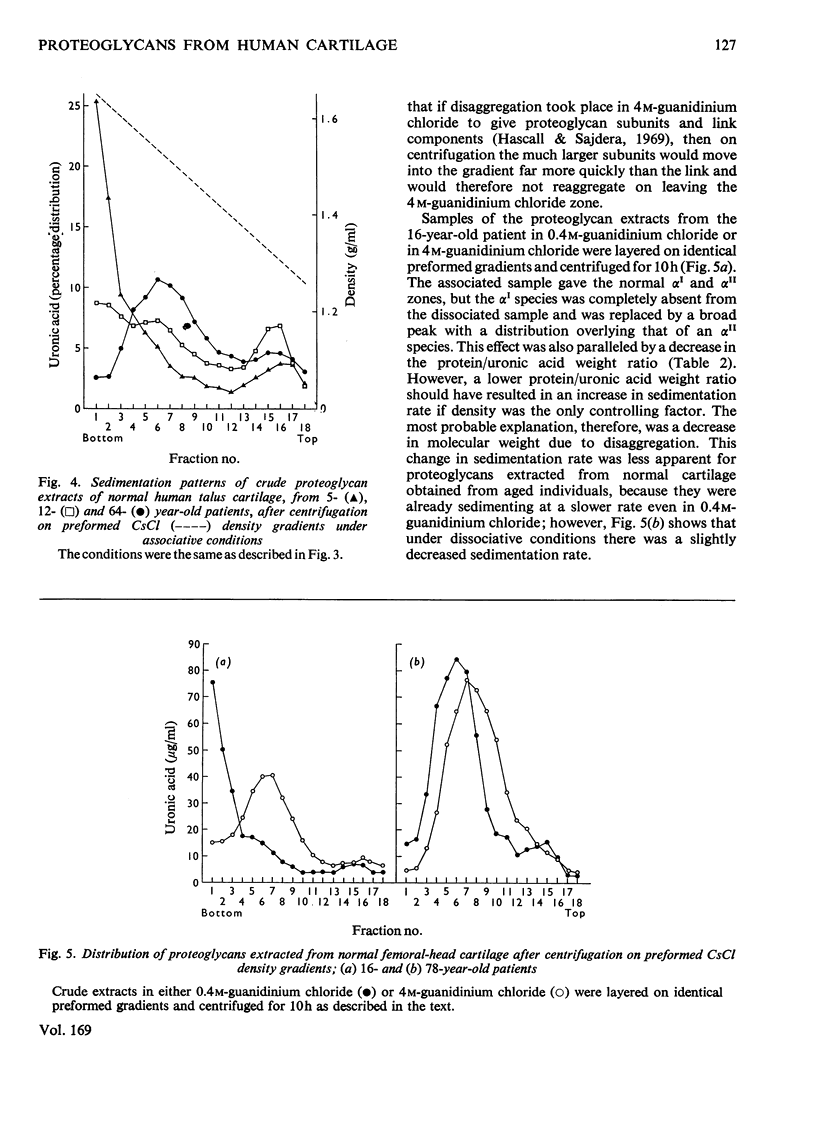
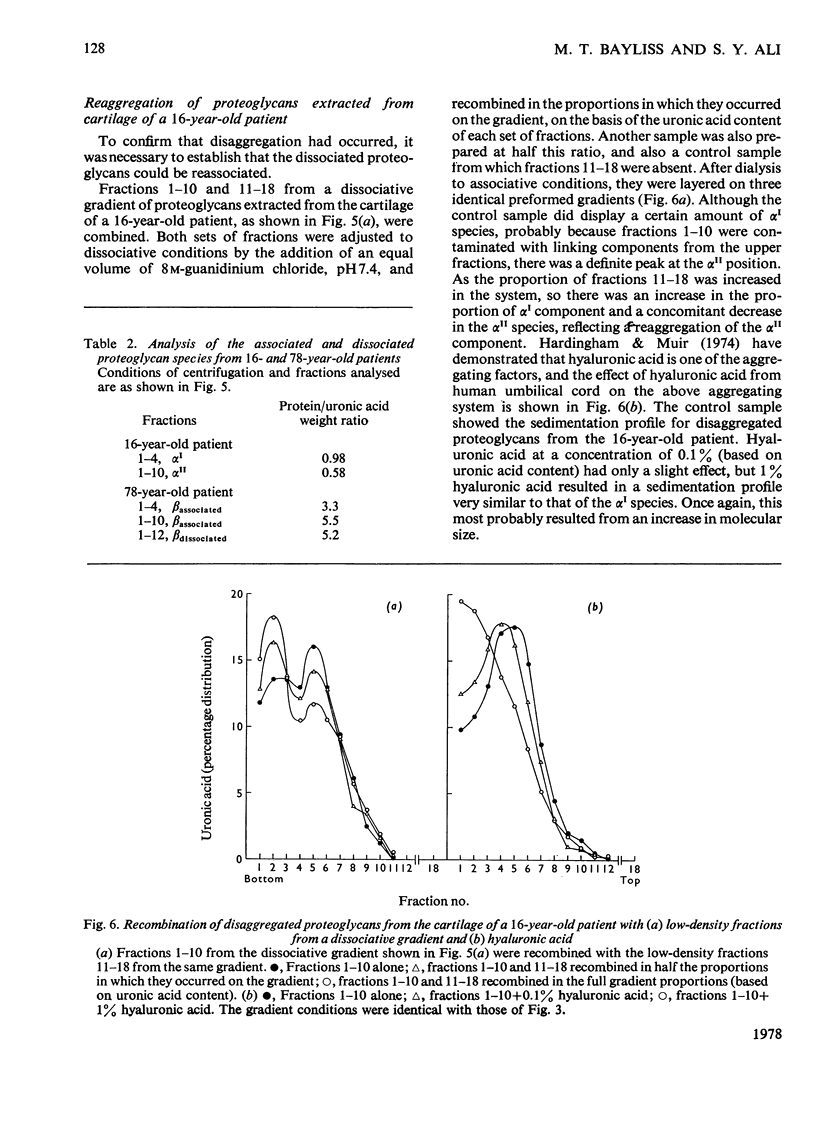
Proteoglycans were extracted from normal human articular cartilage of various ages with 4M-guanidinium chloride and were purified and characterized by using preformed linear CsCl density gradients. With advancing age, there was a decrease in high-density proteoglycans of low protein/uronic acid weight ratio and an increase in the proportion of lower-density proteoglycans, richer in keratan sulphate and protein. Proteoglycans of each age were also shown to disaggregate in 4M-guanidinium chloride and at low pH and to reaggregate in the presence of hyaluronic acid and/or low-density fractions. Osteoarthrotic-cartilage extracts had an increased content of higher-density proteoglycans compared with normal cartilage of the same age, and results also suggested that these were not mechanical or enzymic degradation products, but were possibly proteoglycans of an immature nature.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y. A quantitative method for estimation of acid polysaccharides in blood. Biochim Biophys Acta. 1969 May 6;177(3):641–643. [PubMed] [Google Scholar]
- Altman R. D., Pita J. C., Howell D. S. Degradation of proteoglycans in human osteoarthritic cartilage. Arthritis Rheum. 1973 Mar-Apr;16(2):179–185. doi: 10.1002/art.1780160207. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- ELLIS D. A. A new universal buffer system. Nature. 1961 Sep 9;191:1099–1100. doi: 10.1038/1911099a0. [DOI] [PubMed] [Google Scholar]
- GERBER B. R., FRANKLIN E. C., SCHUBERT M. Ultracentrifugal fractionation of bovine nasal chondromucoprotein. J Biol Chem. 1960 Oct;235:2870–2875. [PubMed] [Google Scholar]
- Gregory J. D. Multiple aggregation factors in cartilage proteoglycan. Biochem J. 1973 Jun;133(2):383–386. doi: 10.1042/bj1330383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- LAURENT T. C., BJOERK I., PIETRUSZKIEWICZ A., PERSSON H. ON THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. II. THE TRANSPORT OF GLOBULAR PARTICLES THROUGH HYALURONIC ACID SOLUTIONS. Biochim Biophys Acta. 1963 Oct 29;78:351–359. doi: 10.1016/0006-3002(63)91645-7. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., PIETRUSZKIEWICZ A. The effect of hyaluronic acid on the sedimentation rate of other substances. Biochim Biophys Acta. 1961 May 13;49:258–264. doi: 10.1016/0006-3002(61)90125-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lohmander S., Hjerpe A. Proteoglycans of mineralizing rib and epiphyseal cartilage. Biochim Biophys Acta. 1975 Sep 8;404(1):93–109. doi: 10.1016/0304-4165(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971 Aug;50(8):1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashburn T. A., Jr, Hoffman P., Hsu D. S. The effect of cations in equilibrium gradient centrifugation of mucopolysaccharides. Biochim Biophys Acta. 1974 Sep 5;362(2):366–374. doi: 10.1016/0304-4165(74)90229-3. [DOI] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- RONDLE C. J., MORGAN W. T. The determination of glucosamine and galactosamine. Biochem J. 1955 Dec;61(4):586–589. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg L., Hellmann W., Kleinschmidt A. K. Macromolecular models of proteinpolysaccharides from bovine nasal cartilage based on electron microscopic studies. J Biol Chem. 1970 Aug 25;245(16):4123–4130. [PubMed] [Google Scholar]
- Rosenberg L., Johnson B., Schubert M. Proteinpolysaccharides from human articular and costal cartilage. J Clin Invest. 1965 Oct;44(10):1647–1656. doi: 10.1172/JCI105271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAPLAND C. G. STUDIES ON A HUMAN THYROID PROTEASE. J Med Lab Technol. 1964 Jan;21:1–20. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Simůnek Z., Muir H. Changes in the protein-polysaccharides of pig articular cartilage during prenatal life, development and old age. Biochem J. 1972 Feb;126(3):515–523. doi: 10.1042/bj1260515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strider W., Pal S., Rosenberg L. Comparison of proteoglycans from bovine articular cartilage. Biochim Biophys Acta. 1975 Jan 30;379(1):271–281. doi: 10.1016/0005-2795(75)90030-6. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]



