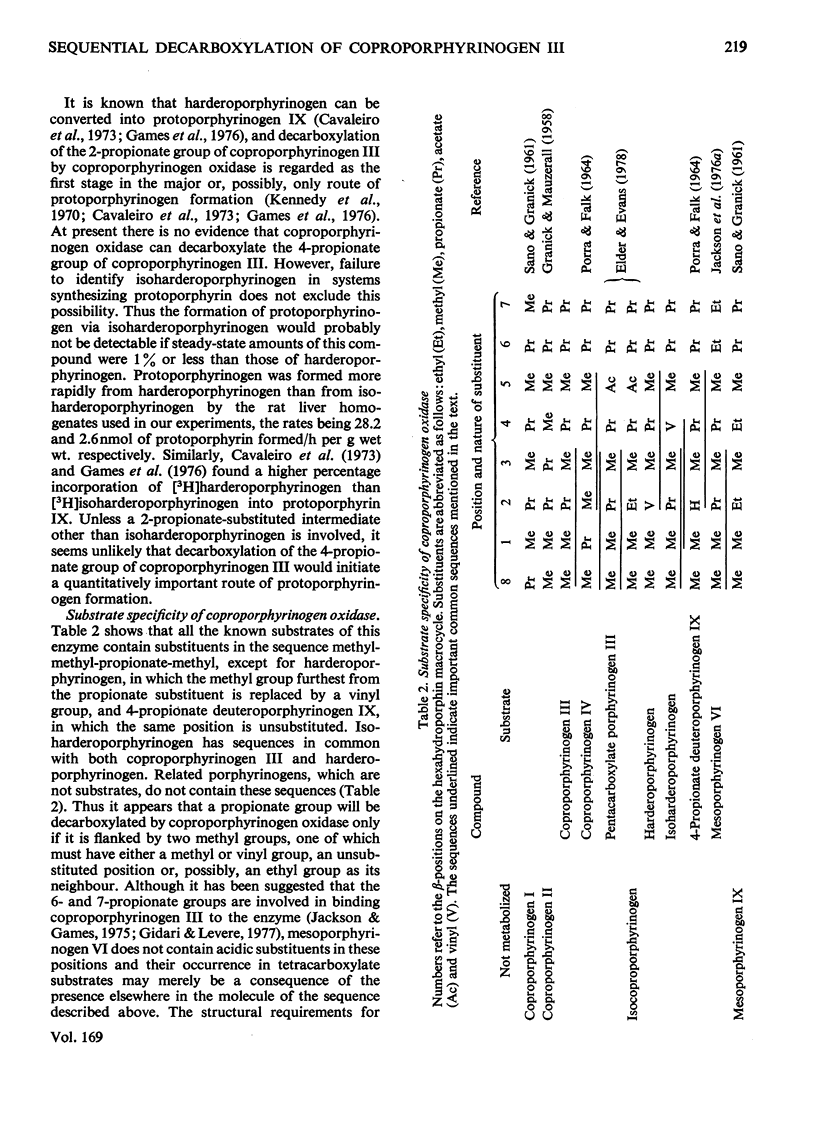
Abstract
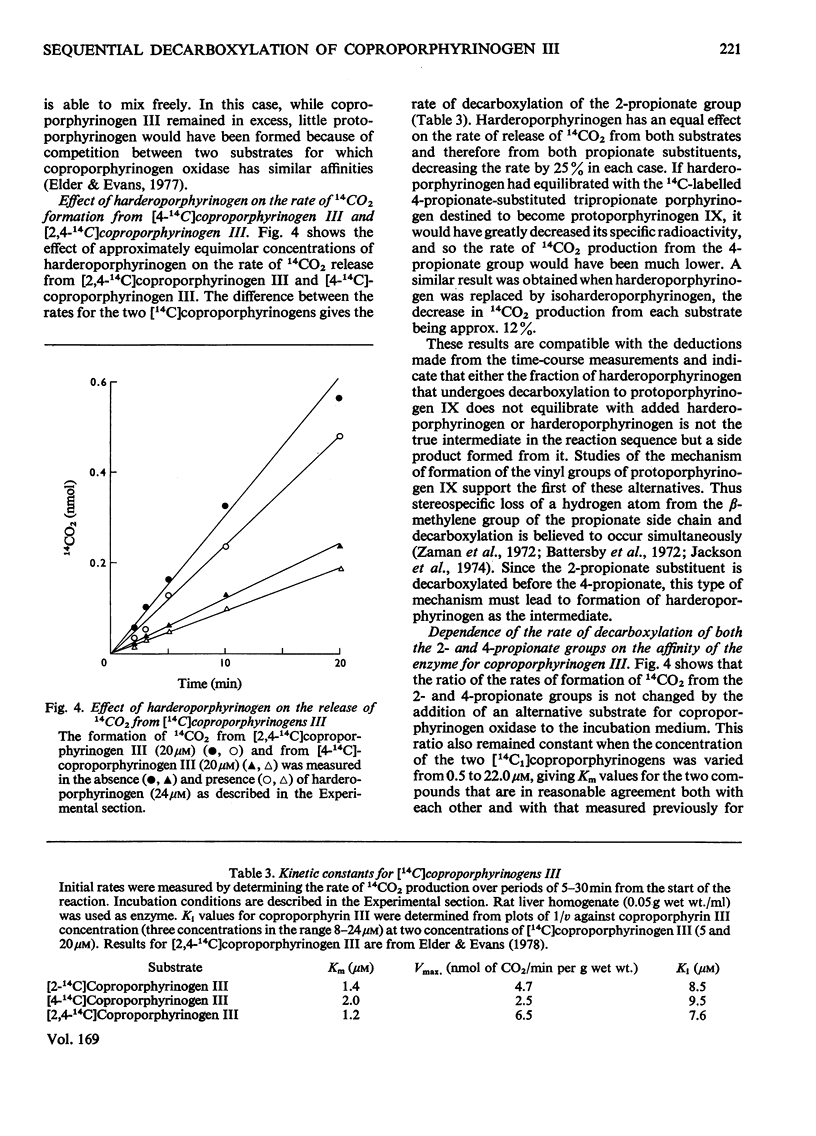
Coproporphyrinogen oxidase (EC 1.3.3.3) catalyses the oxidative decarboxylation of the 2- and 4-propionate substituents of coproporphyrinogen III to form protoporphyrinogen IX. A 4-propionate-substituted porphyrinogen, harderoporphyrinogen, which is also a substrate for coproporphyrinogen oxidase, is formed during the reaction. Synthetic [14C]coproporphyrinogens III, specifically labelled in the carboxyl carbon atoms of either the 2- or 4-propionate substituents, were used to measure the rate of decarboxylation of each substituent by rat liver coproporphyrinogen oxidase. The experimental results, together with the recognition that in all known substrates of coproporphyrinogen oxidase only those propionate groups flanked by a specific arrangement of substituents are decarboxylated, indicate that the 4-propionate group of coproporphyrinogen III cannot be attacked until the 2-propionate group has been decarboxylated. Production of 14CO2 from the substrate labelled in the 2-propionate group therefore measures the formation of harderoporphyrinogen, whereas 14CO2 from the 4-propionate-labelled substrate measures protoporphyrinogen IX formation. The rate of harderoporphyrinogen formation is about twice that of protoporphyrinogen, and this ratio is unchanged by varying the concentration of coproporphyrinogen III or by competitive inhibition of the enzyme. When coproporphyrinogen III is present in an excess, two fractions of harderoporphyrinogen can be distinguished. One accumulates during the reaction, and the other, which is destined to become protoporphyrinogen IX, does not equilibrate with added harderoporphyrinogen. It is suggested that both decarboxylations take place at the same active centre, which becomes temporarily inaccessible to coproporphyrinogen III and added harderoporphyrinogen, and that the molecule rotates after the first decarboxylation to allow the second to take place.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Couch P. W., Games D. E., Jackson A. H. Synthetic and biosynthetic studies of porphyrins. Part 1. Synthesis of the "S-411" porphyrin obtained from meconium. J Chem Soc Perkin 1. 1976;(23):2492–2501. doi: 10.1039/p19760002492. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O. A radiochemical method for the measurement of coproporphyrinogen oxidase and the utilization of substrates other than coproporphyrinogen III by the enzyme from rat liver. Biochem J. 1978 Jan 1;169(1):205–214. doi: 10.1042/bj1690205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder G. H., Evans J. O., Matlin S. A. The effect of the porphyrogenic compound, hexachlorobenzene, on the activity of hepatic uroporphyrinogen decarboxylase in the rat. Clin Sci Mol Med. 1976 Jul;51(1):71–80. doi: 10.1042/cs0510071. [DOI] [PubMed] [Google Scholar]
- Evans N., Jackson A. H., Matlin S. A., Towill R. High-performance liquid chromatographic analysis of porphyrins in clinical materials. J Chromatogr. 1976 Sep 29;125(1):345–355. doi: 10.1016/s0021-9673(00)93830-5. [DOI] [PubMed] [Google Scholar]
- Frydman R. B., Frydman B. Formation of uroporphyrinogen IV during the chemical dimerization of 2-aminomethyl-3',3'-carboxymethyl-4,4' (beta-carboxyethyl) dipyrrylmethane. FEBS Lett. 1975 Apr 1;52(2):317–322. doi: 10.1016/0014-5793(75)80834-9. [DOI] [PubMed] [Google Scholar]
- Gidari A. S., Levere R. D. Enzymatic formation and cellular regulation of heme synthesis. Semin Hematol. 1977 Apr;14(2):145–168. [PubMed] [Google Scholar]
- Jackson A. H., Games D. E., Couch P., Jackson J. R., Belcher R. B., Smith S. G. Conversion of coproporphyrinogen 3 to protoporphyrin IX. Enzyme. 1974;17(1):81–87. doi: 10.1159/000459311. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Games D. E. The later stages of porphyrin biosynthesis. Ann N Y Acad Sci. 1975 Apr 15;244:591–602. doi: 10.1111/j.1749-6632.1975.tb41556.x. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Sancovich H. A., Ferramola A. M., Evans N., Games D. E., Matlin S. A., Elder G. H., Smith S. G. Macrocyclic intermediates in the biosynthesis of porphyrins. Philos Trans R Soc Lond B Biol Sci. 1976 Feb 5;273(924):191–206. doi: 10.1098/rstb.1976.0009. [DOI] [PubMed] [Google Scholar]
- Kennedy G. Y., Jackson A. H., Kenner G. W., Suckling C. J. Isolation, structure and synthesis of a tricarboxylic porphyrin from the harderian glands of the rat. FEBS Lett. 1970 Jan 15;6(1):9–12. doi: 10.1016/0014-5793(70)80027-8. [DOI] [PubMed] [Google Scholar]
- Kushner J. P., Barbuto A. J., Lee G. R. An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity. J Clin Invest. 1976 Nov;58(5):1089–1097. doi: 10.1172/JCI108560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner J. P., Steinmuller D. P., Lee G. R. The role of iron in the pathogenesis of porphyria cutanea tarda. II. Inhibition of uroporphyrinogen decarboxylase. J Clin Invest. 1975 Sep;56(3):661–667. doi: 10.1172/JCI108136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORRA R. J., FALK J. E. Protein-bound porphyrins associated with protoporphyrin biosynthesis. Biochem Biophys Res Commun. 1961 Jun 28;5:179–184. doi: 10.1016/0006-291x(61)90106-1. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Falk J. E. The enzymic conversion of coproporphyrinogen 3 into protoporphyrin 9. Biochem J. 1964 Jan;90(1):69–75. doi: 10.1042/bj0900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]


