Abstract
Large non-pedunculated colorectal polyps ≥20 mm (LNPCPs) constitute approximately 1% of all colorectal polyps and present a spectrum of risks, including overt and covert submucosal invasive cancer (T1 colorectal cancer (CRC)). Importantly, a curative resection may be achieved for LNPCPs with superficial T1 CRC (T1a or T1b <1000 µm into submucosa), if an enbloc R0 excision (clear margins) with favourable histology is achieved (ie, absence of high-grade tumour budding, lympho-vascular invasion, and poor differentiation). Thus, while consensus recommendations advocate for endoscopic resection as the primary treatment option for LNPCPs, thorough optical assessment is imperative for selecting the most suitable ER strategy. In this review, we highlight the critical components of optical evaluation that assist in predicting the risk of T1 CRC, including morphology (Paris and LST classifications), surface pit/vascular pattern (JNET and Kudo classifications), and lesion location. Different resection modalities, including endoscopic submucosal dissection and endoscopic mucosal resection are discussed, along with important considerations that may influence the resection strategy of choice, such as access to the LNPCP and submucosal fibrosis.
Keywords: endoscopic mucosal resection, endoscopic submucosal dissection, large non-pedunculated colorectal polyps
Introduction
Endoscopic resection (ER) stands as the cornerstone in the management of benign colorectal polyps, directly contributing to a reduction in the incidence of colorectal cancer (CRC).1 The vast majority of these polyps (>90%) lack advanced pathology, are smaller than 10 mm, and can be effectively removed through cold-snare polypectomy (CSP).2 However, large non-pedunculated colorectal polyps ≥20 mm (LNPCPs) constitute approximately 1% of all colorectal polyps and present a spectrum of risks, including overt and covert submucosal invasive cancer (T1 CRC). Although consensus recommendations advocate for endoscopic resection (ER) as the primary treatment option for LNPCPs, supported by robust evidence highlighting its superior safety and cost-effectiveness compared to surgery,3–5 thorough optical assessment is imperative for selecting the most suitable ER technique for these lesions.
This is especially crucial as the scope of ER indications has expanded from benign polyps to those harbouring T1 CRC, which may potentially be curable through endoscopic means with careful en-bloc resection, and if horizontal and vertical margins are clear (R0 excision), and the histology is favourable. Favourable histology (low-risk T1 CRC) includes the absence of high-grade tumour budding, lympho-vascular invasion, and poor differentiation. For patients with T1a and T1b CRC plus low-risk histological features, the lymph node and metastases risk (LNM) is 0-0.8% and 2.9%–5.9%, respectively, with a collective recurrence risk of 1.2%.6 Conversely, in patients with T1a or T1b CRC with unfavourable histology, the LNM risk increases to 3.7%–23.9% with a recurrence risk of 9.5% (without salvage surgery).6 Therefore, R0 resection of concerning lesions is paramount to provide accurate histological staging, whilst also offering a chance of curative resection for low-risk T1 CRC lesions. To achieve an en-bloc R0 excision, endoscopic submucosal dissection (ESD) often supersedes the conventional endoscopic mucosal resection (EMR) technique. Furthermore, in instances where the risk of lymph node metastasis (LNM) is deemed significant, ESD does not preclude adjuvant surgery with lymph node resection, and thus can also be considered a staging procedure.
Thus, meticulous endoscopic evaluation and characterization of LNPCPs become indispensable in ensuring the selection of the most appropriate ER technique for each lesion. While distinguishing between benign colorectal polyps and advanced colorectal cancers infiltrating the muscularis propria is generally straightforward, identifying those with T1 CRC poses a greater challenge due to the heterogeneity in size and morphology of LNPCPs. In this review, we aim to elucidate the decision-making process behind piecemeal and en-bloc ER strategies, delineate various ER techniques, outline important adjunctive therapies, and discuss post-resection surveillance protocols.
Piecemeal versus En-bloc resection
Optical evaluation of the colorectal lesion
To guide any ER approach, accurate assessment and diagnosis of the colorectal lesion are of primary importance. An endoscopists optical evaluation should include the lesion’s size, location, morphology, granularity, microvascular, and surface pit patterns. These assessments can be achieved using high-definition white light examination (HD-WLE), narrow band imaging (NBI), or blue-light imaging (BLI) combined with image magnification.
Accurate optical evaluation can help the endoscopist predict histology and identify overt (presence of optical features of T1b CRC or deeper) or covert (absence of overt optical features of CRC, but other high-risk features may be present) cancer. If high-risk features of T1 CRC are detected, en-bloc resection should be performed (features are discussed below). If the risk of T1 CRC is deemed low, piecemeal endoscopic mucosal resection (pEMR) is suitable.
First, lesion gross morphologic assessment is simple and incredibly informative. There are 2 primary endoscopic morphologic classification systems: (A) Paris classification7 and (B) laterally spreading tumour (LST) classification8:
-
(A) Paris classification describes 3 major polyp morphologies:
(1) Polypoid: 0-Ip (pedunculated) or 0-Is (sessile)
(2) Non-polypoid: 0-IIa (minor elevation) or 0-IIb (flat) or 0-IIc (minor depression)
(3) Excavated: 0-III (ulcerated).
-
(B) LST (defined as: colonic polyps that are >10 mm with either a 0-IIa or 0-Is morphology that extend laterally along the colonic wall). Two distinct LST phenotypes with 4 subtypes are described:
(1) LST-granular (LST-G; nodular surface appearance):
(1) Homogeneous LST-G.
(2) Mixed LST-G (LST-MG).
(2) LST-non-granular (LST-NG; smooth surface appearance):
(3) LST-NG pseudo-depressed (PD).
(4) LST-NG flat elevated (FE).
There is now robust data that describes the risk of submucosal invasion based on lesion size and morphology. Homogeneous LST-G colonic lesions are considered to have the lowest risk of T1 CRC (<2%, regardless of size), followed by LST-NG (FE) lesions (10.4% in lesions ≥2 cm).9,10 In contrast, LST-MG polyps have a significantly higher risk of T1 CRC, especially if the polyp size is ≥2 cm and the lesion is in the rectosigmoid location (22.3%–38%).10,11 The highest-risk lesions are LST-NG (PD) polyps and en-bloc resection should always be considered in this lesion phenotype (41.4% in lesions ≥2 cm, irrespective of location).11
Similarly, using the Paris classification, colonic polyps with depressed (0-IIc) morphology are strongly associated with T1 CRC (61%).12–15 In 2017, Burgess et al. assessed the morphology of 2277 polyps that were ≥2 cm and found that 0-Is non-granular and 0-IIa+Is non-granular lesions had a substantially higher risk of covert T1 CRC (OR 22.5, 95% CI 7.07–71.6 and OR 14.4, 95% CI 4.53–45.5, respectively).16 More recently, O’Sullivan et al. assessed a similar cohort of Australian patients (n = 2451 LNPCPs) and reaffirmed that depressed (0-IIc) and nodular (0-IIs or IIa-Is) lesions had a far higher risk of T1 CRC when compared with flat lesions (OR 35.7, 95% CI 22.6–56.5 vs. 3.5, 95% CI 2.6–4.9).11 Similar to the data described by D’Amico et al., O’Sullivan et al. also reported that the rectosigmoid location (vs. proximal location) and polyp size ≥4 cm were both associated with an increased risk of T1 CRC (OR 3.19, 95% CI 2.47–4.13 and OR 1.75, 95% CI 1.35–2.26, respectively).11,17
Colonic lesion size, location and/or morphology alone do not have enough discriminant value to reliably predict risk of T1 CRC. Endoscopic surface pattern assessment is of equal importance and can increase the accuracy of colorectal lesion diagnosis. There are 3 primary endoscopic surface pattern classifications: (A) NBI International Colorectal Endoscopic (NICE) classification,18,19 (B) Japanese NBI expert team (JNET)20 and (C) Kudo pit pattern classification21:
-
(A) NICE classification (see Table 1; does not require magnification endoscopy or dye):
(1) Type 1 (serrated class: either hyperplastic or sessile serrated polyp).
(2) Type 2 (adenoma).
(3) Type 3 (deep T1 CRC and overt T1 CRC).
-
(B) JNET classification (see Table 2; requires magnification endoscopy):
(1) JNET 1 (same as NICE Type 1: either hyperplastic or sessile serrated polyp).
(2) JNET 2A (regular vessel and surface pattern: indicative of low-grade dysplasia).
(3) JNET 2B (variable vessel calibre, irregular vessel and surface pattern: indicative of high-grade dysplasia or superficial T1 CRC).
(4) JNET 3 (same as NICE Type 3: deep T1 CRC and overt T1 CRC)
-
(C) Kudo pit pattern classification (see Table 3; requires dye and magnification endoscopy) describes 6 polyp pit patterns:
(1) Type 1: normal colonic mucosa.
(2) Type 2: asteroid appearance (= hyperplastic polyp).
(3) Type IIIS: tubular or round pit smaller than normal pit (= tubular adenoma).
(4) Type IIIL: tubular or round pit larger than normal pit (= tubular adenoma).
(5) Type IV: gyrus/dendritic (= tubulovillous or villous adenoma).
(6) Type VI: irregular arrangement (= neoplastic or invasive).
(7) Type Vn: loss or decrease of pits with amorphous structure (= neoplastic or invasive).
Table 1.
| Variable | Type 1 | Type 2 | Type 3 |
|---|---|---|---|
| Colour | Same or lighter than the background | Brown relative to background | Brown or black relative to background |
| Vessels | None or isolated lacy vessels | Brown vessels surrounding white structures | Had areas of disrupted or missing vessels |
| Surface pattern | Dark or white spots of uniform size | Oval, tubular, or branched white structures | Amorphous or absence of pattern |
| Most likely histology | Hyperplastic or serrated polyps (sessile serrated polyp) | Adenoma to superficial T1 CRC | Deep T1 CRC |
Table 2.
Japanese Narrow Band Imaging Expert Team Classification13.
| Characteristics | Colours | JNET 2A | JNET 2B | JNET 3 |
|---|---|---|---|---|
| Vessel pattern | Invisible | Regular calibre Regular distribution |
Variable calibre, irregular distribution | Loose vessel areas, interruption of thick vessels |
| Surface pattern | Regular dark or white spots similar to surrounding mucosa | Regular tubular or branched or papillary | Irregular or obscure | Amorphous areas |
| Most likely histology | Hyperplastic polyp or sessile serrated polyp | Low-grade dysplasia | High-grade dysplasia or superficial T1 CRC | Deep T1 CRC |
Table 3.
Kudo Pit Pattern Classification14.
| Types | Features | Interpretation |
|---|---|---|
| I | Round, normal | Normal |
| II | Asteroid | Hyperplastic |
| IIIS | Tubular or round pit smaller than normal pit | Tubular adenoma |
| IIIL | Tubular or round pit larger than normal pit | Tubular adenoma |
| IV | Gyrus/dendritic | Tubulovillous or villous adenoma |
| VI | Irregular arrangement | Neoplastic |
| VN | Loss or decrease of pits with amorphous structure | Neoplastic |
Colonic lesions with a JNET 2B surface pattern or Kudo pit pattern type VI carry a higher risk of superficial T1 CRC (features of covert T1 CRC). As such, these lesions should be considered for en-bloc resection irrespective of lesion location, size, or morphology.20 If the colonic polyp is assessed to be NICE Type 3 or have a JNET 3 surface pattern or Kudo pit pattern type Vn pattern (features of overt T1 CRC), a multidisciplinary team review should be undertaken to discuss the benefit of endoscopic versus surgical resection.
Traditionally, the accuracy of optical diagnosis for T1 CRC was thought to be suboptimal. Recently, endoscopic surface pattern assessment of flat (0-IIa) LNPCPs was deemed to be highly accurate.22 Vosko et al., in a cohort of 1583 patients, noted that in Paris 0-IIa LNPCPs, the sensitivity and specificity for predicting cancer was 91% and 96%, respectively. Conversely, surface pattern assessment for T1 CRC in nodular lesions was found to be less accurate (sensitivity 53%, specificity 94%, missed T1 CRC 6%).22 Therefore, in nodular lesions, overall morphology and location are increasingly important. As mentioned above, nodular lesions in the rectosigmoid location carry a higher risk of covert T1 CRC and as such, Paris 0-Is or LST-MG lesions in the left colon should be considered for en-bloc resection irrespective of surface pattern.
Given the complex interaction of LNPCP characteristics when predicting T1 CRC risk, algorithms have been designed with the hope of improving endoscopist identification of covert and overt T1 CRC. A promising algorithm is the Colorectal Neoplasia Endoscopic Classification to Choose the Treatment (CONECCT) classification.23 The CONECCT classification combines covert (macroscopic features) and overt signs of carcinoma (irregular pit or vascular patterns). Both the sensitivity and negative predictive values for T1 CRC were impressively 100%. Therefore, the use of this classification may decrease unnecessary surgery and non-curative piecemeal endoscopic mucosal resection (pEMR). However, the specificity and positive predictive value were both low (26.2% and 11.6%, respectively), and therefore equally this may lead to many ESDs being performed for benign lesions. Importantly, the CONECCT classification does not include location of lesion, which remains an important consideration.
Recently, O’Sullivan et al. also proposed a simplified T1 CRC decision-making algorithm.11 In this Australian cohort, there was a high prevalence of T1 CRC in depressed LNPCPs (62%). It was proposed that in the absence of surface features of deep T1 CRC (and irrespective of location), en-bloc resection should always be performed for this lesion phenotype. For flat LNPCPs, the prevalence of T1 CRC was unsurprisingly low (4.3% granular, 1.8% non-granular) and the algorithm suggests that pEMR is safe and suitable for these lesions irrespective of granularity or location. For nodular lesions, location is proposed as the primary discriminatory variable, as those in the rectosigmoid location carry a high risk of covert T1 CRC and therefore should always be considered for en-bloc resection. Comparatively, for those in the proximal colon, nodular non-granular LNPCPs carry a high risk of T1 CRC (20%), while nodular granular LNPCPs carry a low risk (5%). Therefore, nodular granular LNPCPs are appropriate for pEMR whilst nodular non-granular LNPCPs should undergo en-bloc resection.
In summary, endoscopic features associated with an increased risk of superficial T1 CRC (covert T1 CRC) in the absence of features concerning for deep T1 CRC (overt T1 CRC) should be resected en-bloc. However, for en-bloc resection of colorectal lesions >20 mm, ESD is required. Ultimately, endoscopists should be aware of the endoscopic features associated with superficial T1 CRC and the rationale for en-bloc resection. When identified in the community or in a non-expert centre, these patients should be referred to a dedicated centre with appropriate endoscopic and colorectal surgical expertise.
Standard techniques for endoscopic resection
Endoscopic mucosal resection (EMR)
Endoscopic mucosal resection (EMR) is a highly efficient and refined procedure, one that has been honed over the past decade to become the most embraced primary modality for LNPCP resection, with a striking 2.48% of all colonoscopies in the United States now incorporating EMR.24 Noteworthy advancements, including the adept recognition and management of deep mural injury (DMI),25,26 alongside innovations such as mechanical closure of post-EMR defects in the proximal colon to mitigate the risk of clinically significant post-EMR bleeding,27 have solidified EMR’s position as a safer and more economical option compared to ESD and surgical interventions.5,28–31
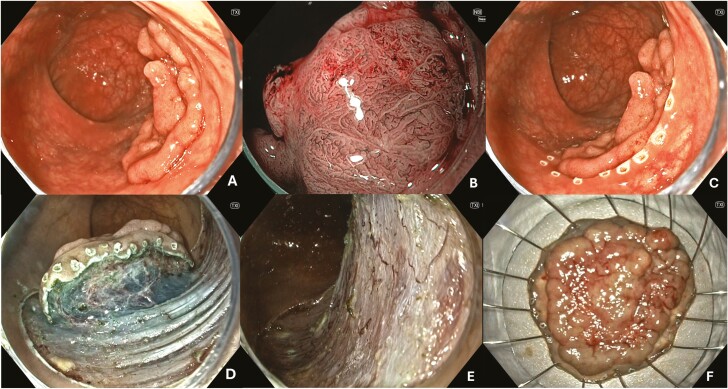
The technique entails several key steps: (1) a submucosal injection of a saline/colloid solution and blue dye, often with diluted epinephrine (referred to as chromo-injectate), (2) systematic snare-based tissue resection, including a 2- to 3-mm margin of normal tissue, (3) transection of the captured tissue in 1–3 pulses of fractionated current, (4) irrigation of the defect post-resection for better identification of residual polypoid tissue and DMI, (5) subsequent resections following the submucosal plane, aligning the snare with the advancing mucosal defect edge and including a 2- to 3-mm margin of submucosal tissue, and (6) repeated submucosal injections every 2–4 resections until complete removal of polypoid tissue is achieved (Figure 1).32–37
Figure 1.
Endoscopic mucosal resection (EMR). (A) Granular, Paris IIa, JNET IIa LNPCP in the tranverse colon. (B) No concerning areas noted on NBI. (C) Submucosal injection performed to lift the lesion. (D) Post-EMR defect. (E) Post-EMR defect following STSC to the margin. (F) Endoscopic clip closure.
Due to its similarities with cold-snare polypectomy (CSP), EMR is easily learned and enables rapid piecemeal resection of extensive LNPCPs, making it particularly suited for adenomatous lesions devoid of overt T1 CRC or with a low risk of covert T1 CRC. Additionally, for LNPCPs measuring up to 25 mm, EMR may facilitate en-bloc R0 excision, serving as an alternative to ESD for such lesions. However, in these cases, ensuring a clear vertical margin typically necessitates a large submucosal injection, employment of a 20 mm or 25 mm snare, and carries a heightened risk of significant DMI (Type III-IV).25,26
Current guidelines also advocate for thermal ablation of the resection margin to reduce the risk of recurrence to 2%–5%.38 This may be achieved with snare-tip soft coagulation (STSC) to create a 3–5 mm rim of ablated tissue (Erbe VIO Soft Coag: 80W, Effect 4; Erbe, Tubingen, Germany). Moreover, for non-lifting or fibrotic areas, adjunctive techniques such as cold avulsion with adjuvant snare-tip soft coagulation (CAST) or hot avulsion can be employed.39 Collectively, these techniques have significantly enhanced the success of EMR for complex lesions, including those previously attempted,40 as well as those situated at challenging locations like the anorectal junction41 or ileocecal valve.42
Endoscopic submucosal dissection (ESD)
Endoscopic Submucosal Dissection (ESD) represents a significant stride in endoscopic innovation, originally conceived as a curative approach for early gastric cancer, yet readily adapted to the colorectum. Theoretically, the size of an LNPCP does not preclude the possibility of achieving an en-bloc R0 excision through ESD. This technique is ideal for lesions exhibiting overt features of T1 CRC or those harboring a heightened risk of covert T1 CRC, offering the potential for organ-sparing, curative resection without undue over-treatment of benign lesions.
While ESD encompasses various iterations, fundamental principles consist of (1) utilization of a distal cap attachment to facilitate access to the submucosal plane, (2) a generous submucosal injection of chromo-injectate to elevate the lesion and expand the submucosal layer, (3) creation of a mucosal incision, and (4) meticulous dissection beneath the lesion within the submucosal plane using a slender (0.3-mm diameter) electrosurgical knife until en-bloc removal is achieved. In certain scenarios, dissection may be performed in a retroflexed position, leveraging enhanced stability and optimizing endoscope alignment parallel to the cutting plane, particularly in rectal procedures (Figure 2).
Figure 2.
Endoscopic submucosal dissection (ESD). (A) Granular Paris IIa+Is, JNET IIa with focal IIb LNPCP in the rectum. (B) Disruption of pit and vascular pattern on NBI. (C) Lesion marked. (D) Submucosal dissection. (E) Final post-ESD defect. (F) Specimen ex-vivo.
Several technical refinements have emerged to bolster safety and efficiency in ESD. The adoption of a variety of traction methods, such as rubber bands,43 multi-loop devices,44 adaptive traction devices,45 and clips with snares or thread, improve the speed of submucosal dissection and reduce the risk of muscle injury or perforation. Innovative approaches to ESD, such as tunnelling or pocket creation are also effective in select lesions.46,47 Paramount to the procedure is the meticulous management of bleeding, especially concerning large submucosal vessels, notably prevalent in the rectum. Vigilant control of bleeding is imperative, as it can hinder visualization, exacerbating the risk of muscle or further vessel injury.
EMR versus ESD
The main consideration between EMR and ESD, comes down to the need for an en-bloc R0 excision, with the objective being complete removal and precise pathological evaluation.48 One of the key benefits of en-bloc resection is that it reduces the risk of incomplete removal of the lesion or incomplete retrieval of all fragments for analysis.48 This is of particular importance for lesions with suspected T1 CRC.
ESD, compared with EMR, has a higher risk of complications including perforation and bleeding. It is also more costly related to more frequent post-procedural hospitalizations. To optimize patient care and resection outcomes, ESD should be performed in a tertiary centre with local expertise and a collaborative colorectal surgical department. It is well documented that the risk of perforation is associated with endoscopist experience (1.4-4.2% vs. 20.4%) and failure of en-bloc resection is also seen in less experienced endoscopists (OR 3.0, 95% CI 1.7–1.8).49,50 The opportunity to obtain expert ESD training in North America is however lacking. There are currently no standardized guidelines for colorectal ESD training nor any recognized credentialing programs. For safe and effective colorectal ESD to be performed, improved access to training is integral in North America.
The utility of an en-bloc resection for benign lesions is less clear. A recent randomized controlled trial (RCT) conducted by a seasoned team in France undertook a comparative analysis of EMR and ESD for LNPCPs measuring 25 mm or larger, with the exclusion of rectal lesions.51 While the recurrence rates were significantly lower in the ESD cohort (0.6%) compared to EMR (5.1%), the absolute rates in both groups were small. Furthermore, recurrences typically manifest as diminutive, low-grade lesions that are readily manageable endoscopically, often with adjunctive techniques such as CAST.52 While there was no difference in the rates of surgical intervention, ESD typically took at least three times longer than EMR, and carried a higher risk of adverse events, such as perforation, delayed bleeding, and post-polypectomy syndrome (35.6% vs. 24.5%).51 Furthermore, in contexts where endoscopy waitlists are extensive, the substantial investment of resources and time in ESD may be deemed inefficient when EMR outcomes remain equivalent.
The opportunity cost associated with protracted ESD procedures, often requiring hospital admission, may inadvertently impede endoscopy access for others, an essential consideration in a global milieu urging optimal resource utilization. Given the predominance of benign lesions in the colon outside the rectum, EMR should persist as the primary choice. It offers a safe, efficient, cost-effective, outpatient-centred approach, effectively mitigating opportunity costs. Nevertheless, for select cases, ESD is an invaluable asset essential for optimizing patient outcomes. Lesions exhibiting endoscopic evidence of superficial T1 CRC or those harboring a high risk for covert T1 CRC, particularly bulky rectal lesions, carry a substantial pretest probability of potentially curable cancer. Thus, ensuring their en-bloc R0 resection through techniques like ESD becomes imperative.53
Cold-snare polypectomy (CSP)
Cold-snare polypectomy (CSP) represents an approach to tissue transection devoid of electrocautery. Employing specialized stiff thin-wire snares (with a wire diameter of ≤0.3 mm), CSP harnesses shearing mechanical forces during snare closure to achieve precise excision. It can be safely executed with or without submucosal injection, although injectate administration can aid in delineating lesion margins. The incorporation of dilute epinephrine serves to mitigate intraprocedural bleeding, facilitating the differentiation between residual polypoid tissue and the expanding resection defect. Echoing the principles of EMR, CSP entails the careful positioning of the snare over the target area, encompassing a healthy margin of surrounding tissue. Upon closure of the snare handle to the point of resistance, the target tissue is captured, followed by complete transection upon confirming appropriate tissue capture. In cases where tissue transection proves challenging, the endoscopist may apply traction against the endoscope by gently pulling on the snare catheter or opt for snare placement revision.
Post-transection, irrigation of the resection defect aids in submucosal expansion and facilitates thorough defect inspection. Oozing from the resection site is anticipated and typically requires no intervention. Cold-snare protrusions, comprising compressed submucosa and muscularis mucosae, are benign entities devoid of adverse outcomes or recurrence implications. Given its shallower depth of resection, CSP finds optimal utility in managing sessile serrated lesions (SSLs), with safe treatment attainable through this approach.54 A recent meta-analysis found that CSP for SSLs carried a technical success rate of 100%, adverse event rate of 2%, and residual or recurrence rate of 3.1%.55
When comparing CSP to EMR for LNPCPs, a recent multicentre RCT from Germany found lower rates of adverse events in the CSP group (1% vs 7.9%, P = .001). However, there was a greater risk of recurrence (23.7% vs 13.8%, P = .020). In multivariable analyses, a lesion diameter of >4cm was an independent predictor for residual adenoma (OR 2.47).56 This is consistent with modelling data, which shows that a 40 mm polyp has a risk of incomplete resection between 40.74% and 60.60%.57 While the results of this RCT are promising, generalizability is limited due to only 30.5% of EMR patients undergoing thermal ablation of the margin, and 36.8% of lesions being SSLs. More recently, an RCT from Australia which enrolled adenomas and where STSC was performed to the EMR-defect margin, found significantly lower rates of recurrence with EMR than CSP (1.1% vs 18.4%, P < .001). As expected, rates of post-EMR bleeding were higher with EMR (7.8% vs 1.1%, P = .034).58 While these RCTs have underscored the superior safety profile of CSP in comparison to EMR, they have also brought to light a significant drawback: a markedly higher risk of recurrence, rendering its application for adenomatous lesions unjustifiable.
Modified techniques for endoscopic resection
Hybrid EMR/ESD
Hybrid EMR/ESD procedures blend components of EMR and ESD components to either achieve an en-bloc resection or aid in piecemeal resection amidst significant submucosal fibrosis. In the case of LNPCPs sized between 25 and 30 mm, hybrid EMR may facilitate quick en-bloc R0 excision as compared to ESD. This involves performing a circumferential mucosal incision around the lesion following chromo-injectate submucosal injection. Once the incision is completed, the lesion often contracts towards the centre. This facilitates direct snare placement within the submucosal plane surrounding the lesion and enables tissue capture beneath the lesion. Piecemeal hybrid EMR may also be beneficial for larger lesions with fibrosis, such as those in a background of ulcerative colitis. In a similar fashion, a circumferential mucosal incision exposes the submucosal plane around the entire LNPCP, facilitating snare placement and resection, albeit in this case in a piecemeal fashion (Figure 3). Hybrid ESD primarily refers to salvage completion EMR in the event that ESD is unable to be completed, often due to severe fibrosis or poor access.59 In a recent meta-analysis, Hybrid ESD was still able to achieve an en-bloc resection rate of 81.6%. Complications, recurrences, and need for surgery occurred in 7.7 %, 4.5%, and 3.6% respectively.
Figure 3.
Hybrid and underwater EMR of a dysplastic lesion in a patient with ulcerative colitis. (A) Non-granular, Paris IIb, JNET IIa descending colon LNPCP. (B) Same lesion visualized with NBI. (C) Lesion marked with diathermic markings then submucosal injection performed. (D) Circumferential incision using the tip of the snare. (E) Resection with underwater EMR. (F) Final defect following hybrid/underwater EMR.
Underwater EMR
This technique involves the instillation of saline to cover the lesion and/or associated colonic segment. Due to the ensuing buoyancy, LNPCPs float away from the muscularis propria. By doing so, this approach enables the en-bloc R0 excision of LNPCPs up to 30 mm in size, without elevating the risk of DMI. In a recent meta-analysis of 7 RCTs with 1458 patients (Underwater-EMR: 739, Conventional-EMR: 719), the pooled rate of en-bloc resection was significantly higher with U-EMR vs C-EMR (70.2% vs 58.1%). R0 resection rates were also higher with U-EMR (58.1% vs 44.6%). While there was no difference in perforation or bleeding, recurrence at surveillance colonoscopy was significantly lower with U-EMR than with C-EMR (RR 0.62).60 However, these studies did not utilize thermal ablation of the margin as part of C-EMR, and therefore were not comparing U-EMR to the accepted standard of care (EMR with thermal ablation of the margin).
Endoscopic intermuscular dissection (EID)
Endoscopic intermuscular dissection (EID) entails dissection between the circular and longitudinal muscularis propria layers, primarily performed in the rectum below the peritoneal reflection. It can be utilized in patients with extensive fibrosis or with suspected deep T1 CRC who are poor surgical candidates.61 In a cohort study of 67 patients, the rates of overall technical success, R0 resection, and curative resection were 96%, 81%, and 45%, respectively. Only minor adverse events occurred in 8 patients (12 %).62 Although the rate of curative resection was high (45%), their definition included all T1 lesions, not just those <1000 μm in depth into the submucosa. This is a controversial area, with Japanese guidelines reporting a depth of submucosal invasion ≥1000 μm as a risk factor prompting surgery.63 Furthermore, ESGE considers sm2/sm3 tumours (≥1000 μm) as high-risk lesions. However, they suggest that following an en-bloc R0 resection of a rectal lesion meeting the single high-risk criterion of submucosal invasion deeper than 1000 μm, options other than surgery could be discussed.64 While further studies are needed to demonstrate its utility in this setting, EID may emerge as a definitive procedure for frail and elderly T1 CRC patients who are non-operative candidates.
Endoscopic full-thickness resection (EFTR)
Endoscopic full-thickness resection (EFTR) methods encompass various techniques, including intentional full-thickness EMR, ESD extending into full-thickness defect, or use of a full-thickness resection device (FTRD). These are typically utilized in the rectum, below the peritoneal reflection, where closure is typically unnecessary. In other (intraperitoneal) colonic locations, closure with clips or endoscopic suturing devices is necessary. FTRD is often limited to lesions close to 15 mm in size. It involves suctioning or pulling the lesion into a cap, including the muscularis propria, followed by clip closure and snare resection over the clip. Similar to EID, it is reserved for cases with suspected deeper T1 CRC or severe fibrosis. Overall clinical success has been shown to be up to 91%, technical success 83%, and R0 excision 81%.65
Important considerations when planning and performing endoscopic resection
The decision between piecemeal and en-bloc resection strategies may also be intricately influenced by various lesion-related characteristics, including challenging access to the LNPCP. This may be due to endoscope-related issues (eg, looping or angulation) or certain anatomical sites (eg, anorectal junction, ileocecal valve, appendiceal orifice, or peri-anastomosis). Although these factors may render ESD unsafe or infeasible, they also confer a higher risk of technical failure, adverse events, and adenoma recurrence with EMR, as highlighted by the SMSA score, which considers lesion access and site among other factors.66
Access
Poor access often complicates attempts at endoscopic resection. Techniques aimed at enhancing access include patient repositioning (left lateral, supine, right lateral, prone), and abdominal pressure application. Changing to a gastroscope may improve flexibility and passage through tight angulations, facilitate retroflexion, and enables devices to exit the channel at a different orientation than a colonoscope. Other methods to improve access include instillation of saline to elevate a lesion, utilization of traction (see above), or use of commercially available overtube devices such as Lumendi67 or Pathfinder68 to prevent looping and provide scope stability.
Complex anatomical locations
While EMR has demonstrated efficacy at anatomically challenging locations such as the anorectal junction,41 ileocecal valve,42 appendiceal orifice,69 and anastomoses,70 data on ESD is still emerging. Regardless, special considerations are warranted when approaching LNPCPs at these sites.
Anorectal junction (ARJ)
The ARJ is located within 2 cm of the pectineal line.71 Endoscopic resection at the ARJ is technically challenging due to the presence of distinctive anatomic and physiologic characteristics,72 including unique innervation and lymphovascular supply secondary to a rectal venous plexus. Thus, specific measures are required to limit pain and bleeding from resecting over the dentate line and haemorrhoidal vessels. There is also a theoretical risk of systemic bacteraemia because of direct drainage into the systemic circulation.73 Surgery is ideally avoided in this area due to the risk of impairing anal function, which can significantly influence the patient’s quality of life.74 The chromo-injectate in this location should include a local anaesthetic to mitigate peri and post-procedural anorectal pain. Another consideration is the risk of stricture formation due to the small luminal diameter in this area, reaching up to 74.2% in cases with an ER defect ≥90% of circumference.75 In these cases, topical nitrates should be considered to reduce anal spasms, steroid to reduce the risk of fibrosis, and facilitation of early endoscopic dilation is key. Despite the challenges, the ARJ location should not preclude either EMR or ESD, especially considering the feasibility of retroflexed views that offer endoscope stability.
Ileocecal valve (ICV)
Lesions involving the ICV may extend into the terminal ileum, necessitating meticulous assessment due to difficulties in distinguishing adenomatous tissue from normal villiform ileal mucosa. Employing a distal cap attachment and initiating resection within the terminal ileum are favourable approaches. In cases of ileal infiltration, resection should begin within the ileum, followed by the anterior and posterior angles of the ICV lips. A limited submucosal injection is recommended in the distal terminal ileum and lips of the ICV because overexpansion of the submucosa may obstruct the lumen and cause the snare to “bounce” off the surface of the valve. A small (10- or 15-mm diameter), stiff, thin-wire snare is preferred at this location because of the relatively small luminal diameter. At the end of the resection, thermal ablation to mitigate the risk of recurrence should be considered, however, the ileal margin avoided due to the risk of stricture formation. When performed systematically in this manner, the technical success of EMR in this region can be upwards of 93.9%, and recurrence as low as (4.6%).42 Although these are not Western studies, a Japanese group has reported their outcomes for ESD performed for LNPCPs involving the ICV.76–78 They demonstrated an en-bloc resection rate of 95% and R0 excision rate of 89%.78 In cases of terminal ileal involvement, a hybrid approach can also be utilized, as a mucosal incision performed with a snare-tip or ESD knife within the terminal ileum, can aid in margin orientation and facilitate polypoid tissue protrusion out into the caecum.
Appendiceal orifice (AO)
Adenomatous lesions of the appendiceal orifice may be effectively managed with EMR. An underwater approach is often preferable to facilitate AO opening and easing EMR execution.79 In one study assessing outcomes of EMR at the AO, complete clearance of visible adenoma was achieved in 92.6% of cases. Reassuringly, there were no cases of post-EMR appendicitis. Of these patients, 91% avoided surgery to longest follow-up. Prior appendectomy status assessment is crucial for AO lesions. In cases without a prior appendectomy, where the distal margin LNPCP cannot be cannot be visualized or if more than 50% of the circumference of the orifice is involved, surgery (appendectomy) should be considered.69 In cases with a prior appendectomy, ESD may be an alternative, as it facilitates a deeper resection, with studies from Japan demonstrating curative resection rates of 70.4%.80
Anastomoses
Anastomotic lesions pose challenges due to associated scarring and fibrosis. Adjunctive techniques are frequently required for these lesions (see below). In a small case series from Australia, peri anastomotic LNPCPs were removed successfully by EMR, but with the majority (90%) requiring CAST. While DMI type II was frequent (40%), there were no cases of DMI III-V, nor any series adverse events. Reassuringly, at 6-month surveillance, there was no recurrence.70
Addressing submucosal fibrosis
Submucosal fibrosis significantly impacts resection outcomes across all ER modalities. Prior resection attempts or the presence of tattoo should raise suspicion of fibrosis,81 necessitating adjunctive techniques for complete resection.
Hot avulsion and CAST
Hot avulsion utilizes biopsy forceps with electrocautery to remove residual adenomatous tissue that is not amenable to snare capture whilst providing thermal ablation at the time of avulsion. In 2014, Veerappan et al reported on the safety and efficacy of hot avulsion in adjunct to snare polypectomy for non-lifting colonic polyps in 20 patients.82 In this cohort, the rate of recurrence was reported to be 15%, however, recurrences were all successfully treated with repeat hot avulsion. In a more recent study of hot avulsion, the rates of recurrence (17.52%) were similar to a cohort of patients that did not require hot avulsion post-EMR (16.02%; P = 0.76).83 While rates of recurrence appear high, this was performed in an era where thermal ablation to the post-EMR defect was not routinely performed. More recently, CAST became an alternative to hot avulsion and involves cold-avulsion of residual adenomatous tissue with a biopsy forceps, followed by snare-tip soft coagulation of the avulsed area. Although it may be laborious and best reserved for small areas of residual adenoma, it has been reported to be highly effective. For instance, in a recent study of previously attempted lesions, CAST was employed in 46.2% of cases, with overall EMR technical success reaching 95.6% and recurrence rate of 7.8%.40 It is important to note that the process of CAST renders it difficult to assess the underlying muscle for injury (DMI type II) and thus any such areas should be managed with targeted clip closure.84 Further studies comparing CAST and hot avulsion are required to determine the most optimal technique for the management of small areas of residual adenoma.
Submucosal release
In scenarios featuring submucosal fibrosis with substantial residual polypoid tissue, CAST, and hot avulsion may be cumbersome. Hence, a submucosal release can be performed to facilitate snare capture. The method involves using a cutting current with the snare-tip to dissect through fibrotic bridges. This works by providing access to the submucosal plane adjacent to the residual tissue, and ultimately facilitates snare-capture. It is important to ensure that an additional submucosal lift with chromo-injectate is performed to avoid inadvertent DMI. While this technique is increasingly being utilized, there is no literature highlighting its effectiveness.
Surveillance and follow-up
After EMR and ESD, surveillance typically entails serial assessments at 6 months (SC1), 18 months (SC2), and 36 months (SC3) post-resection. ESD exhibits near-zero recurrence rates, whereas the recurrence rates following EMR with thermal margin ablation are around 5%. The authors of a recent EMR versus ESD RCT suggested to initiate surveillance colonoscopy at 36 months (SC3) post-ESD, given the minimal recurrence risk.51 Similarly, a recent study from Australia shows that it may be possible to skip to the 36-month surveillance (SC3) post-EMR if SC1 reveals no recurrence, as a clear scar at SC1 is durable at SC2.85 We suggest that SC1 remains vital post-ESD, as up to 16% of patients will have synchronous disease, which is often overlooked or not attended due during the index EMR or ESD.86 This underscores the importance of a thorough assessment via the initial 6-month surveillance colonoscopy (SC1).
Recurrence management, typically modest upon SC1 assessment, warrants similar endoscopic resection techniques as delineated in this review. Modalities typically utilized include EMR with adjunct techniques such as CAST, hot avulsion, and submucosal release. CSP, due to its superficial depth of resection, is often inadequate, while ESD is frequently unnecessary. Recent studies have demonstrated that recurrence can be managed in a single session, with subsequent recurrence rates remaining low.52
In instances of non-curative resections, patients typically require surgical intervention and/or adjuvant chemo-radiotherapy. Comprehensive assessment through imaging modalities such as a CT CAP and MRI pelvis become imperative to evaluate for loco-regional and distant disease extent. Comparatively, in non-operative candidates, a conservative endoscopic surveillance approach may be utilized. In a recent meta-analysis of 2961 T1 CRC patients, disease-specific survival was similar at 5 years.87 Thus, in comorbid non-operative candidates, a non-curative resection may not influence survival.
Supplementary material
Supplementary material is available at Journal of the Canadian Association of Gastroenterology online.
Contributor Information
Sunil Gupta, Division of Gastroenterology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, ON M5B 1W8, Canada; Westmead Hospital, Department of Gastroenterology and Hepatology, Sydney, NSW 2145, Australia.
Tony He, Division of Gastroenterology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, ON M5B 1W8, Canada.
Jeffrey D Mosko, Division of Gastroenterology, Department of Medicine, The Center for Advanced Therapeutic Endoscopy and Endoscopic Oncology, St. Michael’s Hospital, Toronto, ON M5B 1W8, Canada.
Author contributions
Study concept and design: Jeffrey D Mosko. Drafting of the manuscript: Sunil Gupta, Tony He. Critical revision of the manuscript for important intellectual content: Sunil Gupta, Tony He, Jeffrey D. Mosko.
Supplement sponsorship
This article appears as part of the supplement “27th Anniversary Key Topics in Gastroenterology in 2024.” The symposium and this supplement were funded by grants from the following sponsors:
Platinum: Abbvie Canada, Janssen Inc, Pfizer Canada, Takeda Canada, Fresenius-Kabi
Silver: Boston Scientific, Ferring Pharmaceuticals, Organon, Eli Lilly and Company
Conflicts of Interest
S.G. and T.E.: No conflicts of interest. J.D.M.: Speaker Honouraria/Consulting Fees from Boston Scientific, Pendopharm, Medtronic, Fuji.
In addition to this COI statement, ICMJE disclosure forms have been collected for all co-authors and can be accessed as supplementary material.
Data availability
There are no data associated with this review.
References
- 1. Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366(8):687–696. https://doi.org/ 10.1056/NEJMoa1100370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Repici A, Hassan C, Vitetta E, et al. Safety of cold polypectomy for< 10 mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44(1):27–31. https://doi.org/ 10.1055/s-0031-1291387 [DOI] [PubMed] [Google Scholar]
- 3. Ferlitsch M, Hassan C, Bisschops R, et al. Colorectal polypectomy and endoscopic mucosal resection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline–Update 2024. Endoscopy. 2024;56(07):516–545. https://doi.org/ 10.1055/a-2304-3219 [DOI] [PubMed] [Google Scholar]
- 4. Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions: recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020;115(3):435–464. https://doi.org/ 10.14309/ajg.0000000000000555 [DOI] [PubMed] [Google Scholar]
- 5. Kaltenbach T, Anderson JC, Burke CA, et al. Endoscopic removal of colorectal lesions—recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158(4):1095–1129. https://doi.org/ 10.1053/j.gastro.2019.12.018 [DOI] [PubMed] [Google Scholar]
- 6. Ito T, Eishi Y, Kobayashi D, Akashi T, Koike M, Ohashi K. A risk stratification for nodal metastasis in T1 colorectal cancer after successful therapeutic endoscopy. Gastrointest Endosc. 2022;96(1):131–134. https://doi.org/ 10.1016/j.gie.2022.02.041 [DOI] [PubMed] [Google Scholar]
- 7. Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58(6):S3–S43. [DOI] [PubMed] [Google Scholar]
- 8. Okamoto T, Tanaka S, Haruma K, et al. Clinicopathologic evaluation on colorectal laterally spreading tumor (LST). Jpn J Gastroenterol. 1996;93(2):83–89. [PubMed] [Google Scholar]
- 9. Facciorusso A, Antonino M, Di Maso M, Barone M, Muscatiello N. Non-polypoid colorectal neoplasms: classification, therapy and follow-up. World J Gastroenterol. 2015;21(17):5149–5157. https://doi.org/ 10.3748/wjg.v21.i17.5149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68(4):S3–S47. [DOI] [PubMed] [Google Scholar]
- 11. O’Sullivan T, Craciun A, Byth K, et al. A simplified algorithm to evaluate the risk of submucosal invasive cancer in large (≥ 20 mm) nonpedunculated colonic polyps. Endoscopy. 2024;56(8):596–604. https://doi.org/ 10.1055/a-2282-4794 [DOI] [PubMed] [Google Scholar]
- 12. Diebold MD, Samalin E, Merle C, et al. Colonic flat neoplasia: frequency and concordance between endoscopic appearance and histological diagnosis in a French prospective series. Am J Gastroenterol. 2004;99(9):1795–1800. https://doi.org/ 10.1111/j.1572-0241.2004.40236.x [DOI] [PubMed] [Google Scholar]
- 13. dos Santos CEO, Malaman D, Mönkemüller K, et al. Prevalence of non‐polypoid colorectal neoplasms in southern Brazil. Dig Endosc. 2015;27(3):361–367. [DOI] [PubMed] [Google Scholar]
- 14. Rembacken BJ, Fujii T, Cairns A, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. The Lancet. 2000;355(9211):1211–1214. https://doi.org/ 10.1016/s0140-6736(00)02086-9 [DOI] [PubMed] [Google Scholar]
- 15. Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55(11):1592–1597. https://doi.org/ 10.1136/gut.2005.087452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burgess NG, Hourigan LF, Zanati SA, et al. Risk stratification for covert invasive cancer among patients referred for colonic endoscopic mucosal resection: a large multicenter cohort. Gastroenterology. 2017;153(3):732–742.e1. https://doi.org/ 10.1053/j.gastro.2017.05.047 [DOI] [PubMed] [Google Scholar]
- 17. D’Amico F, Amato A, Iannone A, et al. ; Bowell Group. Risk of covert submucosal cancer in patients with granular mixed laterally spreading tumors. Clin Gastroenterol Hepatol. 2021;19(7):1395–1401. https://doi.org/ 10.1016/j.cgh.2020.07.024 [DOI] [PubMed] [Google Scholar]
- 18. Hayashi N, Tanaka S, Hewett DG, et al. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78(4):625–632. https://doi.org/ 10.1016/j.gie.2013.04.185 [DOI] [PubMed] [Google Scholar]
- 19. Hewett DG, Kaltenbach T, Sano Y, et al. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143(3):599–607.e1. https://doi.org/ 10.1053/j.gastro.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 20. Sumimoto K, Tanaka S, Shigita K, et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85(4):816–821. https://doi.org/ 10.1016/j.gie.2016.07.035 [DOI] [PubMed] [Google Scholar]
- 21. Kudo S-E, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44(1):8–14. https://doi.org/ 10.1016/s0016-5107(96)70222-5 [DOI] [PubMed] [Google Scholar]
- 22. Vosko S, Shahidi N, Sidhu M, et al. Optical evaluation for predicting cancer in large nonpedunculated colorectal polyps is accurate for flat lesions. Clin. Gastroenterol. Hepatol.. 2021;19(11):2425–2434.e4. https://doi.org/ 10.1016/j.cgh.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 23. Brule C, Pioche M, Albouys J, et al. The COlorectal NEoplasia Endoscopic Classification to Choose the Treatment classification for identification of large laterally spreading lesions lacking submucosal carcinomas: A prospective study of 663 lesions. United European Gastroenterol J. 2022;10(1):80–92. https://doi.org/ 10.1002/ueg2.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jessica XY, Lin JL, Oliver M, et al. Trends in EMR for nonmalignant colorectal polyps in the United States. Gastrointest Endosc. 2020;91(1):124–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Burgess NG, Bassan MS, McLeod D, Williams SJ, Byth K, Bourke MJ. Deep mural injury and perforation after colonic endoscopic mucosal resection: a new classification and analysis of risk factors. Gut. 2017;66(10):1779–1789. https://doi.org/ 10.1136/gutjnl-2015-309848 [DOI] [PubMed] [Google Scholar]
- 26. Holt BA, Jayasekeran V, Sonson R, Bourke MJ. Topical submucosal chromoendoscopy defines the level of resection in colonic EMR and may improve procedural safety (with video). Gastrointest Endosc. 2013;77(6):949–953. https://doi.org/ 10.1016/j.gie.2013.01.021 [DOI] [PubMed] [Google Scholar]
- 27. Gupta S, Sidhu M, Shahidi N, et al. Effect of prophylactic endoscopic clip placement on clinically significant post-endoscopic mucosal resection bleeding in the right colon: a single-centre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;7(2):152–160. https://doi.org/ 10.1016/s2468-1253(21)00384-8 [DOI] [PubMed] [Google Scholar]
- 28. Bahin FF, Heitman SJ, Rasouli KN, et al. Wide-field endoscopic mucosal resection versus endoscopic submucosal dissection for laterally spreading colorectal lesions: a cost-effectiveness analysis. Gut. 2018;67(11):1965–1973. https://doi.org/ 10.1136/gutjnl-2017-313823 [DOI] [PubMed] [Google Scholar]
- 29. Ahlenstiel G, Hourigan LF, Brown G, et al. ; Australian Colonic Endoscopic Mucosal Resection (ACE) Study Group. Actual endoscopic versus predicted surgical mortality for treatment of advanced mucosal neoplasia of the colon. Gastrointest Endosc. 2014;80(4):668–676. https://doi.org/ 10.1016/j.gie.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 30. Jayanna M, Burgess NG, Singh R, et al. Cost analysis of endoscopic mucosal resection vs surgery for large laterally spreading colorectal lesions. Clin Gastroenterol Hepatol.. 2016;14(2):271–278. https://doi.org/ 10.1016/j.cgh.2015.08.037 [DOI] [PubMed] [Google Scholar]
- 31. Ferlitsch M, Moss A, Hassan C, et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2017;49(3):270–297. https://doi.org/ 10.1055/s-0043-102569 [DOI] [PubMed] [Google Scholar]
- 32. Klein A, Bourke MJ. How to perform high-quality endoscopic mucosal resection during colonoscopy. Gastroenterology. 2017;152(3):466–471. https://doi.org/ 10.1053/j.gastro.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 33. Moss A, Bourke MJ, Williams SJ, et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140(7):1909–1918. https://doi.org/ 10.1053/j.gastro.2011.02.062 [DOI] [PubMed] [Google Scholar]
- 34. Jideh B, Bourke MJ. How to perform wide-field endoscopic mucosal resection and follow-up examinations. Gastrointest Endosc Clin N Am. 2019;29(4):629–646. https://doi.org/ 10.1016/j.giec.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 35. Klein A, Bourke MJ. Advanced polypectomy and resection techniques. Gastrointest Endosc Clin N Am. 2015;25(2):303–333. https://doi.org/ 10.1016/j.giec.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 36. Bourke MJ, Bhandari P. How I remove polyps larger than 20 mm. Gastrointest Endosc. 2019;90(6):877–880. https://doi.org/ 10.1016/j.gie.2019.08.031 [DOI] [PubMed] [Google Scholar]
- 37. Bourke MJ, Bhandari P. How I remove polyps larger than 20 mm. Endoscopy. 2019;51(12):1151–1154. https://doi.org/ 10.1055/a-0999-5427 [DOI] [PubMed] [Google Scholar]
- 38. Klein A, Tate DJ, Jayasekeran V, et al. Thermal ablation of mucosal defect margins reduces adenoma recurrence after colonic endoscopic mucosal resection. Gastroenterology. 2019;156(3):604–613.e3. https://doi.org/ 10.1053/j.gastro.2018.10.003 [DOI] [PubMed] [Google Scholar]
- 39. Tate DJ, Bahin FF, Desomer L, Sidhu M, Gupta V, Bourke M. Cold-forceps avulsion with adjuvant snare-tip soft coagulation (CAST) is an effective and safe strategy for the management of non-lifting large laterally spreading colonic lesions. Endoscopy. 2018;41(01):52–62. [DOI] [PubMed] [Google Scholar]
- 40. Shahidi N, Vosko S, Gupta S, et al. Previously attempted large nonpedunculated colorectal polyps are effectively managed by endoscopic mucosal resection. Am J Gastroenterol. 2021;116(5):958–966. https://doi.org/ 10.14309/ajg.0000000000001096 [DOI] [PubMed] [Google Scholar]
- 41. Shahidi N, Sidhu M, Vosko S, et al. Endoscopic mucosal resection is effective for laterally spreading lesions at the anorectal junction. Gut. 2020;69(4):673–680. https://doi.org/ 10.1136/gutjnl-2019-319785 [DOI] [PubMed] [Google Scholar]
- 42. Vosko S, Gupta S, Shahidi N, et al. Impact of technical innovations in endoscopic mucosal resection in the treatment of large nonpedunculated polyps involving the ileocecal valve (with video). Gastrointest Endosc. 2021;94(5):959–968.e2. https://doi.org/ 10.1016/j.gie.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 43. Jacques J, Charissoux A, Legros R, et al. Double-clip counter-traction using a rubber band is a useful and adaptive tool for colonic endoscopic submucosal dissection. Endoscopy. 2018;50(2):179–181. https://doi.org/ 10.1055/s-0043-122596 [DOI] [PubMed] [Google Scholar]
- 44. Suzuki Y, Tanuma T, Nojima M, et al. Multiloop as a novel traction method in accelerating colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;91(1):185–190. https://doi.org/ 10.1016/j.gie.2019.08.042 [DOI] [PubMed] [Google Scholar]
- 45. Masgnaux LJ, Grimaldi J, Rivory J, et al. Endoscopic submucosal dissection assisted by adaptive traction: results of the first 54 procedures. Endoscopy. 2024;56(3):205–211. https://doi.org/ 10.1055/a-2109-4350 [DOI] [PubMed] [Google Scholar]
- 46. Cecinato P, Lucarini M, Azzolini F, Bassi F, Sassatelli R. Endoscopic submucosal tunnel dissection vs conventional endoscopic submucosal dissection for large colorectal neoplasms: a single-centre retrospective study. Tech Coloproctol. 2023;27(4):317–323. https://doi.org/ 10.1007/s10151-022-02732-8 [DOI] [PubMed] [Google Scholar]
- 47. Yamashina T, Nemoto D, Hayashi Y, et al. Prospective randomized trial comparing the pocket-creation method and conventional method of colorectal endoscopic submucosal dissection. Gastrointest Endosc. 2020;92(2):368–379. https://doi.org/ 10.1016/j.gie.2020.02.034 [DOI] [PubMed] [Google Scholar]
- 48. Probst A, Ebigbo A, Märkl B, et al. Endoscopic submucosal dissection for early rectal neoplasia: experience from a European center. Endoscopy. 2017;49(3):222–232. https://doi.org/ 10.1055/s-0042-118449 [DOI] [PubMed] [Google Scholar]
- 49. Imai K, Hotta K, Yamaguchi Y, et al. Preoperative indicators of failure of en bloc resection or perforation in colorectal endoscopic submucosal dissection: implications for lesion stratification by technical difficulties during stepwise training. Gastrointest Endosc. 2016;83(5):954–962. https://doi.org/ 10.1016/j.gie.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 50. Kim ER, Chang DK. Management of complications of colorectal submucosal dissection. Clin Endosc. 2019;52(2):114–119. https://doi.org/ 10.5946/ce.2019.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jacques J, Schaefer M, Wallenhorst T, et al. Endoscopic en bloc versus piecemeal resection of large nonpedunculated colonic adenomas: a randomized comparative trial. Ann Intern Med. 2024;177(1):29–38. https://doi.org/ 10.7326/M23-1812 [DOI] [PubMed] [Google Scholar]
- 52. Tate DJ, Desomer L, Argenziano ME, et al. Treatment of adenoma recurrence after endoscopic mucosal resection. Gut. 2023;72(10):1875–1886. https://doi.org/ 10.1136/gutjnl-2023-330300 [DOI] [PubMed] [Google Scholar]
- 53. Shahidi N, Vosko S, Gupta S, et al. A rectum-specific selective resection algorithm optimizes oncologic outcomes for large nonpedunculated rectal polyps. Clin Gastroenterol Hepatol. 2023;21(1):72–80.e2. https://doi.org/ 10.1016/j.cgh.2022.04.021 [DOI] [PubMed] [Google Scholar]
- 54. Van Hattem WA, Shahidi N, Vosko S, et al. Piecemeal cold snare polypectomy versus conventional endoscopic mucosal resection for large sessile serrated lesions: a retrospective comparison across two successive periods. Gut. 2021;70(9):1691–1697. https://doi.org/ 10.1136/gutjnl-2020-321753 [DOI] [PubMed] [Google Scholar]
- 55. Ding C, Yang J-f, Wang X, et al. Cold EMR vs. Hot EMR for the removal of sessile serrated polyps larger than 10 mm: a systematic review and meta-analysis. BMC Surg. 2024;24(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steinbrück I, Ebigbo A, Kuellmer A, et al. Cold versus Hot Snare Endoscopic Resection of Large Non-Pedunculated Colorectal Polyps (Randomized-controlled German CHRONICLE-trial). Gastroenterology. 2024;167(4):764–777. https://doi.org/ 10.1053/j.gastro.2024.05.013 [DOI] [PubMed] [Google Scholar]
- 57. Cronin O, Kirszenblat D, Forbes N, et al. Geometry of cold snare polypectomy and risk of incomplete resection. Endoscopy. 2024;56(3):214–219. https://doi.org/ 10.1055/a-2184-1609 [DOI] [PubMed] [Google Scholar]
- 58. O’Sullivan T, Cronin O, Hattem AV, et al. Cold vs hot snare endoscopic mucosal resection for large (≥15 mm) flat non-pedunculated colorectal polyps: a randomized controlled trial. Gut. 2024:gutjnl-2024-332807. Epub Ahead of Print. https://doi.org/ 10.1136/gutjnl-2024-332807 [DOI] [PubMed] [Google Scholar]
- 59. Van Langendonck S, Van Heddegem N, Bekaert J, Rasquin K, Dewint P. Hybrid EMR as a salvage technique during colonic EMR. Endoscopy. 2023;55(S 02):140.35688454 [Google Scholar]
- 60. Chandan S, Bapaye J, Khan SR, et al. Safety and efficacy of underwater versus conventional endoscopic mucosal resection for colorectal polyps: systematic review and meta-analysis of RCTs. Endosc Int Open. 2023;11(08):E768–E777. https://doi.org/ 10.1055/a-2117-8327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tribonias G, Komeda Y, Leontidis N, et al. Endoscopic intermuscular dissection (EID) for removing early rectal cancers and benign fibrotic rectal lesions. Tech Coloproctol. 2023;27(12):1393–1400. https://doi.org/ 10.1007/s10151-023-02862-7 [DOI] [PubMed] [Google Scholar]
- 62. Moons LM, Bastiaansen BA, Richir MC, et al. Endoscopic intermuscular dissection for deep submucosal invasive cancer in the rectum: a new endoscopic approach. Endoscopy. 2022;54(10):993–998. [DOI] [PubMed] [Google Scholar]
- 63. Hashiguchi Y, Muro K, Saito Y, et al. ; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25(1):1–42. https://doi.org/ 10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pimentel-Nunes P, Libânio D, Bastiaansen BA, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline–Update 2022. Endoscopy. 2022;54(06):591–622. [DOI] [PubMed] [Google Scholar]
- 65. Mahadev SH, Vareedayah AA, Yuen S, Yuen W, Koller KA, Haber GB. Outcomes of a hybrid technique using EMR and endoscopic full-thickness resection for polyps not amenable to standard techniques (with video). Gastrointest Endosc. 2021;94(2):358–367.e1. https://doi.org/ 10.1016/j.gie.2021.02.009 [DOI] [PubMed] [Google Scholar]
- 66. Sidhu M, Tate DJ, Desomer L, et al. The size, morphology, site, and access score predicts critical outcomes of endoscopic mucosal resection in the colon. Endoscopy. 2018;50(7):684–692. https://doi.org/ 10.1055/s-0043-124081 [DOI] [PubMed] [Google Scholar]
- 67. Ismail MS, Bahdi F, Mercado MO, et al. ESD with double-balloon endoluminal intervention platform versus standard ESD for management of colon polyps. Endosc Int Open. 2020;8(10):E1273–E1279. https://doi.org/ 10.1055/a-1226-6372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wei MT, Hwang JH, Watson RR, Park W, Friedland S. Novel rigidizing overtube for colonoscope stabilization and loop prevention (with video). Gastrointest Endosc. 2021;93(3):740–749. https://doi.org/ 10.1016/j.gie.2020.07.054 [DOI] [PubMed] [Google Scholar]
- 69. Tate DJ, Desomer L, Awadie H, et al. EMR of laterally spreading lesions around or involving the appendiceal orifice: technique, risk factors for failure, and outcomes of a tertiary referral cohort (with video). Gastrointest Endosc. 2018;87(5):1279–1288.e2. https://doi.org/ 10.1016/j.gie.2017.12.018 [DOI] [PubMed] [Google Scholar]
- 70. Cronin O, Gupta S, Gauci J, et al. Endoscopic resection of large anastomotic polyps is safe and effective. Endoscopy. 2024;56(2):125–130. https://doi.org/ 10.1055/a-2174-2967 [DOI] [PubMed] [Google Scholar]
- 71. Ferreira MF, Marques M, Morais R, Lemmers A, Macedo G, Santos-Antunes J. Endoscopic submucosal dissection is safe and effective for lesions located at the anorectal junction: Analysis from two referral European Centers. GE Port J Gastroenterol. 2024;31(1):41–47. https://doi.org/ 10.1159/000528107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Imai K, Hotta K, Yamaguchi Y, et al. Safety and efficacy of endoscopic submucosal dissection of rectal tumors extending to the dentate line. Endoscopy. 2015;47(6):529–532. https://doi.org/ 10.1055/s-0034-1391078 [DOI] [PubMed] [Google Scholar]
- 73. Holt BA, Bassan MS, Sexton A, Williams SJ, Bourke MJ. Advanced mucosal neoplasia of the anorectal junction: endoscopic resection technique and outcomes (with videos). Gastrointest Endosc. 2014;79(1):119–126. https://doi.org/ 10.1016/j.gie.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 74. Kunitake H, Poylin V. Complications following anorectal surgery. Clin Colon Rectal Surg. 2016;29(01):014–021. https://doi.org/ 10.1055/s-0035-1568145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gupta S, Vosko S, Shahidi N, et al. Endoscopic resection-related colorectal strictures: risk factors, management, and long-term outcomes. Endoscopy. 2023;55(11):1010–1018. https://doi.org/ 10.1055/a-2106-6494 [DOI] [PubMed] [Google Scholar]
- 76. Yoshizaki T, Toyonaga T, Tanaka S, et al. Feasibility and safety of endoscopic submucosal dissection for lesions involving the ileocecal valve. Endoscopy. 2016;48(07):639–645. https://doi.org/ 10.1055/s-0042-102783 [DOI] [PubMed] [Google Scholar]
- 77. Andrisani G, Fukuchi T, Antonelli G, et al. Superficial neoplasia involving the Ileocecal valve: Clinical outcomes of endoscopic submucosal dissection. Dig Liver Dis. 2021;53(7):889–894. https://doi.org/ 10.1016/j.dld.2021.03.005 [DOI] [PubMed] [Google Scholar]
- 78. Tanaka H, Oka S, Kunihiro M, et al. Endoscopic submucosal dissection for tumors involving the ileocecal valve with extension into the terminal ileum: a multicenter study from the Hiroshima GI Endoscopy Research Group. Surg Endosc. 2023;37(2):958–966. https://doi.org/ 10.1007/s00464-022-09542-x [DOI] [PubMed] [Google Scholar]
- 79. Binmoeller KF, Hamerski CM, Shah JN, Bhat YM, Kane SD. Underwater EMR of adenomas of the appendiceal orifice (with video). Gastrointest Endosc. 2016;83(3):638–642. https://doi.org/ 10.1016/j.gie.2015.08.079 [DOI] [PubMed] [Google Scholar]
- 80. Hotta K, Osera S, Shinoki K, et al. Feasibility of endoscopic submucosal dissection for cecal tumors involving the ileocecal valve or appendiceal orifice. J Gastroenterol Hepatol. 2022;37(8):1517–1524. https://doi.org/ 10.1111/jgh.15872 [DOI] [PubMed] [Google Scholar]
- 81. Shahidi N, Gupta S, Whitfield A, et al. Simple optical evaluation criteria reliably identify the post-endoscopic mucosal resection scar for benign large non-pedunculated colorectal polyps without tattoo placement. Endoscopy. 2022;54(2):173–177. https://doi.org/ 10.1055/a-1469-9917 [DOI] [PubMed] [Google Scholar]
- 82. Veerappan SG, Ormonde D, Yusoff IF, Raftopoulos SC. Hot avulsion: a modification of an existing technique for management of nonlifting areas of a polyp (with video). Gastrointest Endosc. 2014;80(5):884–888. https://doi.org/ 10.1016/j.gie.2014.05.333 [DOI] [PubMed] [Google Scholar]
- 83. Kumar V, Broadley H, Rex DK. Safety and efficacy of hot avulsion as an adjunct to EMR (with videos). Gastrointest Endosc. 2019;89(5):999–1004. https://doi.org/ 10.1016/j.gie.2018.11.032 [DOI] [PubMed] [Google Scholar]
- 84. Bar-Yishay I, Shahidi N, Gupta S, et al. Outcomes of deep mural injury after endoscopic resection: an international cohort of 3717 large non-pedunculated colorectal polyps. Clin Gastroenterol Hepatol. 2022;20(2):e139–e147. https://doi.org/ 10.1016/j.cgh.2021.01.007 [DOI] [PubMed] [Google Scholar]
- 85. O’Sullivan T, Mandarino F, Nascimento C, et al. Impact of margin thermal ablation after endoscopic mucosal resection of large (≥ 20mm) non-pedunculated colonic polyps on long term recurrence. Gastrointest Endosc. 2024;99(6):AB445. [DOI] [PubMed] [Google Scholar]
- 86. O’Sullivan T, Tate D, Sidhu M, et al. The surface morphology of large nonpedunculated colonic polyps predicts synchronous large lesions. Clin Gastroenterol Hepatol. 2023;21(9):2270–2277.e1. https://doi.org/ 10.1016/j.cgh.2023.01.034 [DOI] [PubMed] [Google Scholar]
- 87. Chen Y, Jing W, Chen M, et al. Long-term outcomes of local resection versus surgical resection for high-risk T1 colorectal cancer: a systematic review and meta-analysis. Gastrointest Endosc. 2023;97(6):1016–1030.e14. https://doi.org/ 10.1016/j.gie.2023.02.027 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no data associated with this review.