Abstract
Although Alzheimer disease neuropathologic change (ADNC) is the most common pathology underlying clinical dementia, the presence of multiple comorbid neuropathologies is increasingly being recognized as a major contributor to the worldwide dementia burden. We analyzed 1051 subjects with specific combinations of isolated and mixed pathologies and conducted multivariate logistic regression analysis on a cohort of 4624 cases with mixed pathologies to systematically explore the independent cognitive contributions of each pathology. Alzheimer disease neuropathologic change and limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) were both associated with a primary clinical diagnosis of Alzheimer disease (AD) and were characterized by an amnestic dementia phenotype, while only ADNC associated with logopenic variant primary progressive aphasia (PPA). In subjects with ADNC and comorbid LATE-NC, Lewy body disease, and/or cerebrovascular disease, the clinical phenotype was usually diagnosed during life as “Probable AD.” Conversely, the combination of ADNC with frontotemporal lobar degeneration with TDP-43, progressive supranuclear palsy (PSP), or corticobasal degeneration (CBD) resulted in a mixed clinical picture, with variable features of amnestic dementia, PPA subtypes, behavioral variant FTD, PSP syndrome, and CBD syndrome. These findings elucidate the cumulative effects of mixed pathologies and provide insights into interactions between neurodegenerative pathologies contributing to a variety of clinical dementia presentations.
Keywords: Alzheimer disease neuropathologic change (ADNC), cerebrovascular disease (CVD), frontotemporal lobar dementia (FTLD-TDP), Lewy body dementia (LBD), limbic-predominant age-related TDP-43 encephalopathy (LATE-NC), Pick disease (PiD), primary age-related tauopathy (PART)
INTRODUCTION
Clinical dementia represents a growing public health challenge worldwide. The number of individuals with cognitive impairment is projected to rise from 60 million in 2019 to approximately 150 million by 2050, underscoring the necessity for multifaceted approaches, encompassing both public health planning efforts and the advancement of biomedical research into disease mechanisms, antemortem biomarkers, and development of therapies.1–3 Alzheimer disease neuropathologic change (ADNC) remains the most common underlying pathological finding in patients with clinical dementia and the pathologic prevalence may be much higher than the prevalence of clinical Alzheimer disease (AD).4,5 It has been established, however, that much of clinical dementia results from comorbid diseases. Other relatively common neuropathologic changes, such as cerebrovascular disease (CVD), Lewy body disease (LBD), and limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), are frequently comorbid with ADNC and contribute to cognitive impairment in an additive or synergistic manner.6–17 Various combinations of neurodegenerative pathologies can manifest with a clinical phenotype similar to AD, making it difficult to discern which of these pathologies, and to what degree, contributed to the clinical phenotype. This overlap of neuropathologic changes and clinical phenotypes presents significant challenges in linking specific symptoms to underlying pathologic processes, as well as designing clinically effective disease-specific biomarkers and therapies.
The correlation between autopsy-proven neuropathologic and clinical features has been a frequently studied topic in several large cohorts, including the National Alzheimer’s Coordinating Center (NACC) dataset.9,18–20 We previously analyzed a cohort of 6262 subjects with 0-6 comorbid neuropathologic entities from the NACC database using multivariate logistic regression analysis to better understand the role of specific individual neurodegenerative pathologies on global cognition and specific cognitive domains.21 The most common clinically significant neuropathologies in this cohort were intermediate/high-level ADNC, followed by CVD (including infarcts, hemorrhage, arteriolosclerosis, and white matter rarefaction), LBD, and LATE-NC. That study revealed an additive effect of multiple neuropathologies on cognitive impairment and identified specific neurocognitive tests independently affected by each disorder. Herein, we used a subset of this same cohort to investigate the impact of ADNC, LATE-NC, LBD, CVD, frontotemporal lobar degeneration with TDP-43 (FTLD-TDP), amyotrophic lateral sclerosis (ALS), Pick disease (PiD), progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and argyrophilic grain disease (AGD), as isolated and combined pathologies, on antemortem primary clinical diagnoses, clinical phenotypes, psychiatric and behavioral symptoms, and cognitive domains. These data give further insight into the specific role that each neurodegenerative process plays in cognitive decline, as well as how they may interact in individuals with multiple comorbid disease states.
METHODS
Case selection and exclusion criteria
Data for this study were downloaded with permission from the NACC, established with funding from the National Institute on Aging (NIA) (U01 AG016976) (https://naccdata.org/), which is a widely utilized cohort for cognitive clinicopathologic studies,4,8,15,21–42 as well as investigations on behavioral and psychiatric features of neurodegenerative diseases.43–48 This cohort is sourced from 37 Alzheimer’s Disease Research Centers (ADRCs) located across the United States. A total of 6262 unique cases were identified; this number was reduced to 4624 cases with sufficient neuropathologic and clinical information for analysis (including Clinical Dementia Rating [CDR] Dementia Staging Instrument recorded at the final clinical visit), and last cognitive evaluation performed within 24 months of participant death. We utilized standardized Uniform Data Set (UDS), version 3 variable definitions (https://naccdata.org/data-collection/forms-documentation/uds-3), Neuropathology (NP) Data Set, version 11 variable definitions (https://naccdata.org/data-collection/forms-documentation/np-11), and Genetic Data Set (Gen) variable definitions (https://files.alz.washington.edu/documentation/rdd-genetic-data.pdf) from NACC, as previously described.15,21,49,50
Demographic, genetic, and neuropathologic variables
Demographic, genetic, and neuropathologic data on subjects included in this study are presented in Table 1. The mean time between last clinical assessment and autopsy for the entire cohort was 9.9 ± 0.2 months. Subject age at death was derived from the UDS variable NACCDAGE; sex was assessed with the UDS variable SEX; race was determined from the UDS variable RACE, Hispanic/Latino ethnicity was determined from the variable HISPANIC; and years of education was assessed with the UDS variable EDUC. APOE genotype (ε2/2, ε2/3, ε2/4, ε3/3, ε3/4, ε4/4) was assessed with the variable NACCAPOE. Alzheimer’s Disease Research Center site was determined using the variable NACCADC and date of visit was determined with the variables VISITMO and VISITYR.
Table 1.
Demographic and genetic features of subjects with isolated and combined neuropathologic findings.
| n | Age at death (years) | Sex (M:F) | Education (years) | Race (% White) a | Ethnicity (% Hispanic) |
APOE status
b
|
Time from last exam (months) | ||
|---|---|---|---|---|---|---|---|---|---|
| ≥1 APOE ε2 allele | ≥1 APOE ε4 allele | ||||||||
| Controls/minimal pathology | 22 | 71.6 ± 3.9 | 13:9 | 16.5 ± 0.5 | 95.5% | 0% | 28.6% | 19.0% | 9.1 ± 1.2 |
| ADNC | 126 | 79.4 ± 0.9 | 67:59 | 16.0 ± 0.3 | 95.2% | 2.4% | 10.5% | 55.3% | 9.2 ± 0.6 |
| P-value (compared to control) | — | 0.0043 | 0.6074 | 0.5063 | 0.9588 | 0.4647 | 0.0254 | 0.0023 | 0.9477 |
| LATE-NC | 14 | 82.9 ± 3.1 | 6:8 | 15.9 ± 0.2 | 100% | 0% | 27.3% | 18.2% | 9.4 ± 1.7 |
| P-value (compared to control) | — | 0.0474 | 0.3415 | 0.3622 | 0.4185 | >0.9999 | 0.9381 | 0.9525 | 0.8830 |
| P-value (compared to ADNC) | — | 0.2277 | 0.4635 | 0.8690 | 0.4020 | 0.5595 | 0.1026 | 0.0187 | 0.9158 |
| LBD | 11 | 78.5 ± 2.6 | 9:2 | 15.9 ± 0.2 | 90.9% | 9.1% | 10.0% | 20.0% | 7.8 ± 1.7 |
| P-value (compared to control) | — | 0.2469 | 0.1917 | 0.4150 | 0.6059 | 0.1510 | 0.2477 | 0.9500 | 0.5365 |
| P-value (compared to ADNC) | — | 0.7751 | 0.0668 | 0.8836 | 0.5269 | 0.2049 | 0.9585 | 0.0323 | 0.5049 |
| CVD | 76 | 78.0 ± 1.5 | 44:32 | 18.5 ± 1.4 | 88.2% | 2.6% | 20.6% | 16.2% | 10.6 ± 0.6 |
| P-value (compared to control) | — | 0.0691 | 0.9202 | 0.4480 | 0.3194 | 0.4420 | 0.4436 | 0.7587 | 0.2471 |
| P-value (compared to ADNC) | — | 0.3951 | 0.5136 | 0.0261 | 0.0654 | 0.9116 | 0.0606 | <0.0001 | 0.1224 |
| ADNC + LATE-NC | 35 | 85.7 ± 1.6 | 16:19 | 15.5 ± 0.5 | 91.4% | 5.7% | 6.1% | 36.4% | 10.6 ± 1.1 |
| P-value (compared to control) | — | 0.0003 | 0.3254 | 0.1853 | 0.5624 | 0.2537 | 0.0232 | 0.1743 | 0.3767 |
| P-value (compared to ADNC) | — | 0.0012 | 10:25 | 0.2802 | 0.3920 | 0.3146 | 0.4451 | 0.0558 | 0.2749 |
| ADNC + LBD | 89 | 76.6 ± 1.2 | 53:36 | 16.8 ± 1.0 | 94.4% | 4.5% | 5.0% | 61.3% | 9.6 ± 0.7 |
| P-value (compared to control) | — | 0.1080 | >0.9999 | 0.8830 | 0.8421 | 0.3112 | 0.0013 | 0.0006 | 0.7445 |
| P-value (compared to ADNC) | — | 0.0583 | 0.3538 | 0.3604 | 0.7894 | 0.3898 | 0.1684 | 0.4060 | 0.6659 |
| ADNC + CVD | 203 | 81.2 ± 0.8 | 101:102 | 15.6 ± 0.2 | 93.6% | 4.0% | 10.1% | 58.5% | 9.7 ± 0.5 |
| P-value (compared to control) | — | 0.0006 | 0.4054 | 0.1544 | 0.7318 | 0.3418 | 0.0134 | 0.0006 | 0.7028 |
| P-value (compared to ADNC) | — | 0.1471 | 0.5462 | 0.1816 | 0.5459 | 0.4396 | 0.9072 | 0.5802 | 0.5280 |
| LATE-NC + CVD | 20 | 85.0 ± 2.4 | 10:10 | 16.3 ± 0.6 | 90.0% | 0% | 26.4% | 26.3% | 10.4 ± 1.5 |
| P-value (compared to control) | — | 0.0067 | 0.5544 | 0.7979 | 0.4930 | >0.9999 | 0.8732 | 0.5825 | 0.4988 |
| P-value (compared to ADNC) | — | 0.0239 | 0.7916 | 0.5900 | 0.3443 | 0.4856 | 0.0563 | 0.0194 | 0.4601 |
| LBD + CVD | 18 | 80.8 ± 2.5 | 13:5 | 16.8 ± 0.5 | 100% | 5.6% | 5.6% | 27.8% | 12.8 ± 1.8 |
| P-value (compared to control) | — | 0.0668 | 0.3864 | 0.6769 | 0.3596 | 0.2629 | 0.0619 | 0.5189 | 0.0859 |
| P-value (compared to ADNC) | — | 0.5855 | 0.1282 | 0.1568 | 0.3423 | 0.4433 | 0.5107 | 0.0301 | 0.0388 |
| ADNC + LATE-NC + LBD | 22 | 78.4 ± 1.5 | 15:7 | 19.4 ± 3.8 | 100% | 0% | 10.5% | 73.7% | 7.8 ± 1.2 |
| P-value (compared to control) | — | 0.1111 | 0.5308 | 0.4535 | 0.3117 | >0.9999 | 0.1542 | 0.0005 | 0.4479 |
| P-value (compared to ADNC) | — | 0.6568 | 0.1913 | 0.0392 | 0.2941 | 0.4647 | >0.9999 | 0.1322 | 0.3592 |
| ADNC + LATE-NC + CVD | 69 | 86.7 ± 1.0 | 37:32 | 17.4 ± 1.2 | 94.2% | 0% | 1.6% | 51.6% | 9.0 ± 0.9 |
| P-value (compared to control) | — | <0.0001 | 0.6536 | 0.6767 | 0.8225 | >0.9999 | 0.0001 | 0.0093 | 0.9542 |
| P-value (compared to ADNC) | — | <0.0001 | 0.9521 | 0.1324 | 0.7637 | 0.1965 | 0.0308 | 0.6426 | 0.8490 |
| ADNC + LBD + CVD | 111 | 76.3 ± 1.1 | 58:53 | 16.6 ± 0.8 | 92.8% | 2.7% | 8.3% | 58.3% | 9.6 ± 0.5 |
| P-value (compared to control) | — | 0.1209 | 0.5569 | 0.9562 | 0.6498 | 0.43333 | 0.0096 | 0.0011 | 0.6882 |
| P-value (compared to ADNC) | — | 0.0288 | 0.8871 | 0.4427 | 0.4346 | 0.8661 | 0.5897 | 0.6547 | 0.6145 |
| LATE-NC + LBD + CVD | 2 | 87.0 ± 3.0 | 0:2 | 17.0 ± 1.0 | 100% | 0% | 0% | 0% | 10.0 ± 1.0 |
| P-value (compared to control) | — | 0.2564 | 0.1083 | 0.7723 | 0.7581 | >0.9999 | 0.3793 | 0.4971 | 0.8269 |
| P-value (compared to ADNC) | — | 0.2916 | 0.1352 | 0.5321 | 0.7509 | 0.8252 | 0.6280 | 0.1199 | 0.8674 |
| ADNC + LATE-NC + LBD + CVD | 57 | 84.3 ± 1.1 | 23:34 | 15.9 ± 0.4 | 92.8 | 3.5% | 5.6% | 72.2% | 9.7 ± 0.9 |
| P-value (compared to control) | — | <0.0001 | 0.1338 | 0.4045 | 0.6734 | 0.3735 | 0.0059 | <0.0001 | 0.7140 |
| P-value (compared to ADNC) | — | 0.0016 | 0.1081 | 0.8480 | 0.5237 | 0.6647 | 0.2913 | 0.0356 | 0.6433 |
| PART | 18 | 85.4 ± 2.4 | 8:10 | 15.8 ± 0.3 | 94.4% | 0% | 16.7% | 5.6% | 9.8 ± 1.4 |
| P-value (compared to control) | — | 0.0069 | 0.3561 | 0.2634 | 0.8841 | >0.9999 | 0.3790 | 0.2090 | 0.7048 |
| P-value (compared to ADNC) | — | 0.0199 | 0.4880 | 0.8039 | 0.8895 | 0.5082 | 0.4456 | <0.0001 | 0.7206 |
| FTLD-TDP | 18 | 69.7 ± 2.1 | 11:7 | 16.4 ± 0.6 | 94.4% | 0% | 11.7% | 29.4% | 10.4 ± 1.7 |
| P-value (compared to control) | — | 0.6895 | 21:31 | 0.8979 | 0.8841 | >0.9999 | 0.2064 | 0.4549 | 0.5257 |
| P-value (compared to ADNC) | — | 0.0002 | 12:39 | 0.6291 | 0.8895 | 0.5082 | 0.8775 | 0.0466 | 0.4845 |
| ALS | 2 | 59.5 ± 2.5 | 1:1 | 17.1 ± 1.0 | 100% | 0% | 0% | 50.0% | 12.0 ± 3.0 |
| P-value (compared to control) | — | 0.3697 | 0.8028 | 0.7285 | 0.7581 | >0.9999 | 0.3793 | 0.3106 | 0.4885 |
| P-value (compared to ADNC) | — | 0.0064 | 0.9289 | 0.6464 | 0.7509 | 0.8252 | 0.6280 | 0.8820 | 0.5598 |
| PiD | 22 | 63.9 ± 1.9 | 15:7 | 15.9 ± 0.6 | 95.2% | 4.5% | 25.0% | 25.0% | 10.3 ± 1.7 |
| P-value (compared to control) | — | 0.1300 | 12:44 | 0.4467 | 0.9731 | 0.3004 | 0.7964 | 0.6453 | 0.5672 |
| P-value (compared to ADNC) | — | <0.0001 | 4:35 | 0.8956 | 0.9940 | 0.5347 | 0.0728 | 0.0125 | 0.4930 |
| PSP | 61 | 73.7 ± 1.3 | 36:25 | 16.2 ± 1.4 | 93.2% | 6.6% | 15.1% | 26.4% | 9.9 ± 0.8 |
| P-value (compared to control) | — | 0.5104 | 0.9951 | 0.8991 | 0.7102 | 0.2183 | 0.1820 | 0.5054 | 0.5990 |
| P-value (compared to ADNC) | — | 0.0004 | 0.4515 | 0.8510 | 0.5804 | 0.1584 | 0.3974 | 0.0005 | 0.4961 |
| CBD | 23 | 68.0 ± 1.6 | 14:9 | 15.7 ± 0.5 | 86.4% | 4.3% | 31.6% | 26.3% | 9.7 ± 1.5 |
| P-value (compared to control) | — | 0.3904 | 0.9031 | 0.2644 | 0.2943 | 0.3226 | 0.8358 | 0.5825 | 0.7576 |
| P-value (compared to ADNC) | — | <0.0001 | 0.4957 | 0.6837 | 0.1109 | 0.5915 | 0.0130 | 0.0194 | 0.7464 |
| AGD | 4 | 84.0 ± 4.9 | 1:3 | 15.0 ± 1.5 | 75.0% | 0% | 0% | 25.0% | 7.8 ± 3.4 |
| P-value (compared to control) | — | 0.2037 | 0.2084 | 0.2686 | 0.1579 | >0.9999 | 0.2201 | 0.7850 | 0.6831 |
| P-value (compared to ADNC) | — | 0.3713 | 0.2667 | 0.5588 | 0.0792 | 0.7549 | 0.4936 | 0.2324 | 0.6831 |
Abbreviations: ADNC, Alzheimer disease neuropathologic change; AGD, argyrophilic grain disease; ALS, amyotrophic lateral sclerosis; CBD, corticobasal degeneration; CVD, cerebrovascular disease; FTLD-TDP, frontotemporal lobar degeneration with TDP-43; LATE-NC, limbic-predominant age-related TDP-43 encephalopathy neuropathologic change; LBD, Lewy body disease; PiD, Pick disease; PSP, progressive supranuclear palsy.
Given the paucity of non-White subjects, statistics for the ethnicity column are calculated simply with groups of “White” and “non-White”.
APOE data are not available for all individuals; bold denotes significant at a level of P < .05.
Each neurodegenerative pathology was assessed from NACC NP dataset variables. Alzheimer disease neuropathologic change levels were determined from the NACC NP dataset variable NPADNC; cases with NPADNC 2-3 (“intermediate” to “high” level ADNC) were considered positive for ADNC. In instances where NPADNC was not available, ADNC levels were derived from a combination of Braak stage (NACCBRAA), Thal phase (NPTHAL), and CERAD neuritic plaque (NP) score (NACCNEUR), where possible.21,51–54 In some cases, the exact ADNC level could not be determined but a diagnosis of “not”/“low” or “intermediate”/“high” ADNC could still be established. For example, a case with a NACCBRAA 6, NACCNEUR 3, and unknown NPTHAL correlates with either “intermediate” or “high” ADNC level (NPADNC), and therefore it was included here as positive for ADNC, while a case with NACCBRAA 6 and NACCNEUR 1 with an unknown NPTHAL could correlate with either “low” or “intermediate” ADNC level (NPADNC), and therefore was not included as either ADNC positive or negative for the purposes of this study.51,52 Definite primary age-related tauopathy (PART) was assessed from a combination of NACCBRAA, NPTHAL, and NACCNEUR, as previously described.39,55–57 LBD stage58,59 was assessed using the NACC NP dataset variables NACCLEWY and NPLBOD; cases with limbic (transitional) or diffuse neocortical were included as positive for LBD.
Frontotemporal lobar degeneration with TDP-43, ALS, and LATE-NC were assessed using NACC NP dataset variables NPFTDTDP, NPALSMND, NPTDPA (TDP-43-immunoreactive inclusions in the spinal cord), NPTDPB (TDP-43-immunoreactive inclusions in amygdala), NPTDPC (TDP-43-immunoreactive inclusions in the hippocampus), and NPTDPE (TDP-43-immunoreactive inclusions in neocortex), as previously described, using updated recommendations for distinguishing these neuropathologies.41,60 Cases with a neuropathologic diagnosis of FTLD-TDP and TDP-43-immunoreactive inclusions in the neocortex were included as FTLD-TDP. Cases were assigned LATE-NC stage 0 in the absence of a neuropathologic diagnosis of FTLD-TDP and an absence of TDP-43 immunoreactivity in any region, LATE-NC stage 1 with TDP-43-immunoreactive inclusions in the amygdala only, LATE-NC stage 2 with TDP-43-immunoreactive inclusions in the amygdala and hippocampus but not neocortex, and LATE-NC stage 3 with TDP-43 inclusions in the amygdala, hippocampus, and neocortex and an absence of a diagnosis of FTLD-TDP, ALS, or noted genetic mutations known to be associated with FTLD-TDP or ALS.8,60–62 Similar to ADNC, a case with positive NPTDPB and positive NPTDPC, but absent information for NPTDPE, was included as positive for LATE-NC, as the subject would have either LATE-NC stage 2 or 3. However, an individual with positive NPTDPB but unknown NPTDPC and NPTDPE was not included, as the subject could have LATE-NC stage 1, 2, or 3, depending on the unrecorded TDP-43 variables.
Pick disease was determined with the NACC NP dataset variable NACCPICK. Progressive supranuclear palsy was determined with the NACC NP dataset variable NACCPROG. Corticobasal degeneration was determined with the NACC NP dataset variable NACCCBD. Argyrophilic grain disease was determined with the NACC NP dataset variable NPFTDT5. Aging-related tau astrogliopathy (ARTAG) was assessed with the NACC NP dataset variables NPARTAG and NPATGSEV.42 Cerebrovascular disease was determined using a combination of infarcts/lacunes (NACCINF), hemorrhages/microbleeds (NACCHEM), arteriolosclerosis (NACCARTE), and white matter rarefaction (NPWMR). Cerebral amyloid angiopathy (CAA) was determined with the NACC NP dataset variable NACCAMY. Hippocampal sclerosis was determined with the NACC NP dataset variable NPHIPSCL. Of note, CAA and hippocampal sclerosis were used as covariates for multivariate logistic regression analysis but are not displayed in figures. Cases designated as controls/minimal pathology had ADNC levels of “not” or “low” as well as Braak stage ≤1, LATE-NC stage 0-1, LBD stage 0-1, and no other identified neurodegenerative pathologies.21,52,53,58,61 Cases included as combinations of ADNC, LATE-NC, LBD, and CVD were selected exclusively from cases with sufficient available data for all 4 of these neuropathologic variables. When determining “isolated” pathologies, we included cases with low levels of other pathologies, due to the ubiquity of some relatively mild protein deposits in the vast majority of subjects. For example, a subject with “isolated ADNC” may have LATE-NC restricted to the amygdala (stage 1) or brainstem-only LBD (stage 1). The number of cases positive for each neuropathology included in this study (isolated and total), as well as the number of ADRCs from which each diagnostic category was sourced, are detailed in Table S1. Examples of neuropathologic features are shown in Figure 1.
Figure 1.
Representative neurodegenerative pathology. (A) Alzheimer disease with neurofibrillary tangles in CA1 (p-tau, AT8). (B) Alzheimer disease with neurofibrillary degeneration in the calcarine cortex (p-tau, AT8). (C) Alzheimer disease with β-amyloid deposition in the cerebellar molecular layer (β-amyloid, 6E10). (D) Neocortical cerebral amyloid angiopathy and a neuritic plaque (Thioflavin S). (E) Primary age-related tauopathy with neurofibrillary tangles primarily affecting CA2 (AT8). (F) Lewy bodies in pigmented substantia nigra neurons (LFB/H&E). (G) Limbic stage Lewy body disease with Lewy bodies and Lewy neurites in the CA2 subregion (α-synuclein). (H) TDP-43 inclusions in the cytoplasm of dentate gyrus neurons. (I) TDP-43 inclusions in the cytoplasm of pyramidal cortical neurons. (J) Pick bodies in dentate gyrus neurons (3R tau). (K) Progressive supranuclear palsy with a cortical tufted astrocyte (4R tau). (L) Remote infarct in the basal ganglia (H&E).
Clinical phenotypes and antemortem primary clinical diagnoses
Primary diagnoses at last clinical encounter (within the final 24 months of life) were derived from the UDS datasheet variable NACCETPR, and included primary etiologic diagnoses of AD, dementia with Lewy bodies, multiple system atrophy, PSP, corticobasal degeneration, FTLD with motor neuron disease, FTLD, vascular dementia, unimpaired, and a range of other causes of cognitive impairment (Table S2). Clinical phenotypes were derived from UDS variables and included nonimpaired (NORMCOG), any degree/type of dementia (DEMENTED), amnestic dementia (AMNDEM), posterior cortical atrophy (PCA), any type of primary progressive aphasia (any PPA; NACCPPA), PPA subtype (including semantic PPA, logopenic PPA, and nonfluent/agrammatic PPA; NACCPPAG), behavioral variant FTD (bvFTD; NACCBVFT), Lewy body dementia syndrome (LBDS; NACCLBDS), parkinsonism (PARK), progressive supranuclear palsy syndrome (PSP syndrome; PSP), corticobasal degeneration syndrome (CBD syndrome; CORT), memory impairment (COGMEM), orientation impairment (COGORI), judgment impairment (COGJUDG), language impairment (COGLANG), visuospatial impairment (COGVIS), and attention impairment (COGATTN).
Psychiatric and behavioral variables
Psychiatric symptoms were derived from UDS variables and included delusions in the last month (DEL), delusion severity (DELSEV), hallucinations in the last month (HALL), hallucination severity (HALLSEV), agitation or aggression in the last month (AGIT), agitation or aggression severity (AGITSEV), depression or dysphoria in the last month (DEPD), depression or dysphoria severity (DEPDSEV), anxiety in the last month (ANX), anxiety severity (ANXSEV), elation or euphoria in the last month (ELAT), elation or euphoria severity (ELATSEV), apathy or indifference in the last month (APA), apathy or indifference severity (APASEV), disinhibition in the last month (DISN), disinhibition severity (DISNSEV), irritability or lability in the last month (IRR), irritability or lability severity (IRRSEV), motor disturbance in the last month (MOT), motor disturbance severity (MOTSEV), nighttime behaviors in the last month (NITE), nighttime behaviors severity (NITESEV), appetite and eating problems in the last month (APP), appetite and eating problems severity (APPSEV) (Table S2).
Data analysis
Multivariate logistic regression analyses and correlation analyses were performed with MedCalc (MedCalc Software Ltd), on the set of cases with sufficient minimum clinical and neuropathologic data and death within 24 months of the last clinical encounter (n = 4624). These included subjects with all combinations of neurodegenerative pathology and subjects with some missing neuropathologic and cognitive variables. Alzheimer’s Disease Research Center, year of final clinical evaluation, age, sex, and years of education were used as covariates for this multivariate logistic regression analysis. Phi correlation coefficients for clinical phenotypes were calculated as the partial r for binary variables after adjusting for ADRC, year of final clinical evaluation, age, sex, and level of education in MedCalc. All other statistical analyses were performed with GraphPad Prism version 10 (GraphPad Software, Inc.). Differences in the proportion of sex, race, ethnicity, and APOE status among neuropathologically defined groups were calculated using Fisher’s exact test. Differences between age, education, and time between last clinical exam and subject death were evaluated using multiple t-tests. Statistical significance was set at α = 0.05 and P-values of cognitive/pathologic correlations were adjusted for multiple testing using false discovery rate correction. All graphs were created using GraphPad Prism.
RESULTS
Demographic features of the included cohort
Demographic features of the included cohort according to each combination of neurodegenerative pathology are detailed in Table 1. Subjects with isolated PART were significantly older than control or ADNC subjects and subjects with isolated FTLD-TDP, ALS, PiD, PSP, and CBD were all significantly younger than ADNC subjects. In groups with combined pathologies, those with combined ADNC/LATE-NC, ADNC/LATE/CVD, and ADNC/LATE-NC/LBD/CVD were all significantly older than subjects in the isolated ADNC and control/minimal pathology groups. There were minimal differences in terms of sex, years of education, race, ethnicity, and time between last clinical exam and subject death between each group.
In terms of APOE genetic status, the group of subjects with isolated ADNC had a significantly lower prevalence of APOE ε2 alleles and a significantly higher prevalence of APOE ε4 alleles compared to control subjects (P = .0254 and P = .0023, respectively). Non-ADNC pathology cohorts generally had lower rates of APOE ε4 alleles compared to ADNC subjects, including LATE-NC (P = .0187), LBD (P = .0323), CVD (P < .0001), LATE-NC/CVD (P = .0194), LBD/CVD (P = .0301), PART (P < .0001), FTLD-TDP (P = .0466), PiD (P = .0125), PSP (P = .0005), and CBD (P = .0194), without significant differences compared to control subjects. Subjects with combinations of ADNC and other common neuropathologic findings generally had similar frequencies of ε2 compared to isolated ADNC. Subjects with combined ADNC/LATE-NC/LBD/CVD were significantly more likely to harbor APOE ε4 alleles compared to isolated ADNC (P = .0356) (Table 1).
Clinicopathologic correlations of isolated neuropathologic findings
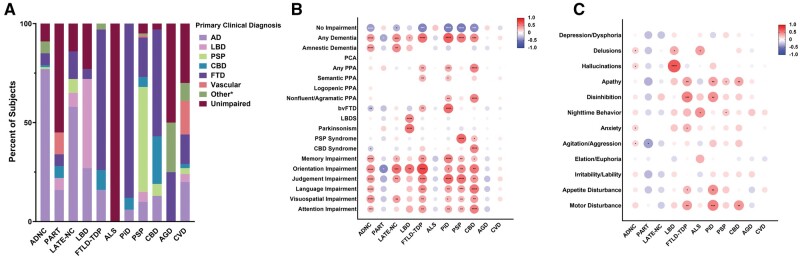
Approximately 77% (97/126) of subjects with autopsy-proven isolated ADNC were given a primary antemortem clinical diagnosis of AD, 45% (5/11) of subjects with isolated LBD were given a primary clinical diagnosis of dementia with Lewy bodies (although some individuals lacked cognitive impairment or displayed an AD phenotype), while 72% (13/18) of subjects with isolated FTLD-TDP and 86% (19/22) of subjects with isolated PiD were given a primary clinical diagnosis of FTD (Figure 2A). Among 4-repeat (4R) tauopathies, roughly half of the isolated PSP group were given a primary diagnosis of clinical PSP (49.2%, 30/61) and roughly half of the subjects with isolated CBD neuropathology were given a primary diagnosis of FTD (47.8%, 11/23). More than half of the isolated PART group were cognitively unimpaired (55.6%, 10/18). In the isolated LATE-NC group, over half of the subjects were given an antemortem primary clinical diagnosis of AD (57.1%, 8/14), with some also showing unimpaired cognitive function or a clinical FTD phenotype.
Figure 2.
Clinical associations with isolated neurodegenerative pathologies. (A) Primary diagnoses of subjects with autopsy-confirmed isolated intermediate- or high-level Alzheimer disease neuropathologic change (ADNC; n = 126), primary age-related tauopathy (PART; n = 18), limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC; n = 14), Lewy body disease (LBD; n = 11), frontotemporal lobe dementia with TDP-43 (FTLD-TDP; n = 18), amyotrophic lateral sclerosis (ALS; n = 2), Pick disease (PiD; n = 22), progressive supranuclear palsy (PSP; n = 61), corticobasal degeneration (CBD; n = 23), argyrophilic grain disease (AGD; n = 4), and cerebrovascular disease (CVD; n = 76). (B) Pairwise correlation between clinical phenotypes and each autopsy-confirmed isolated neuropathology, calculated using Phi correlation coefficients. (C) Pairwise correlation between psychiatric/behavioral symptoms and each autopsy-confirmed isolated neuropathology, calculated using Phi correlation coefficients. *P < .05; **P < .01; ***P < .001; ****P < .0001. The primary diagnosis Other* consists of additional underlying causes for cognitive impairment, detailed in Table S2. Abbreviations: bvFTD, behavioral variant frontotemporal dementia; PCA, posterior cortical atrophy; PPA, primary progressive aphasia.
With regard to more specific clinical phenotypes (Figure 2B), subjects with isolated ADNC demonstrated a strong positive correlation with amnestic dementia (P < .0001), as well as impairment in memory (P < .0001), orientation (P < .0001), judgment (P < .0001), language (P < .0001), visuospatial function (P < .0001), and attention (P = .0003) (Figure 2B). Isolated LATE-NC demonstrated a similar clinical phenotype to AD, correlating with amnestic dementia (P = .0059), as well as impairments in memory (P = .0283), orientation (P = .0006), judgment (P = .0035), and visuospatial function (P = .0059). Eight of the 14 LATE-NC cases had hippocampal sclerosis, but similar clinical correlates were present with and without hippocampal sclerosis in these subjects. Isolated LBD pathology exhibited a strong correlation with LBDS (P < .0001) and Parkinsonism (P < 0.0001), as well as impairment in orientation (P = 0.0018). Semantic variant PPA was significantly associated with isolated FTLD-TDP (P = .0084) and isolated PiD (P = .0241). Nonfluent/agrammatic PPA was associated with isolated PiD (P = .0071) and CBD (P < .0001). Behavioral variant FTD was associated with isolated FTLD-TDP (P = .0018) and isolated PiD (P < .0001). In contrast, isolated PART, ALS, AGD, and CVD demonstrated no definitive cognitive associations and in some cases had trends toward negative correlations with some forms of cognitive impairment. With regard to psychiatric and behavioral symptoms, subjects with isolated LBD had a strong association with hallucinations (P < .0001), while FTLD-TDP and PiD both had strong associations with apathy (P = .0081 and P = .0086, respectively), disinhibition (P = .0002 and P = .0012, respectively), and disturbances in appetite (P = .0460 and P = .0014, respectively) and motor function (P = .0046 and P = .0002, respectively) (Figure 2C). Apathy was also a feature of subjects with isolated PSP (P = .0221) and CBD (P = .0079).
Clinicopathologic correlations of mixed common pathologies
Reflecting the fact that ADNC and CVD are the most common isolated pathologies, a significant proportion of study subjects had concurrent ADNC and/or CVD, in addition to overlapping LATE-NC and/or LBD (Figure 3A and B). Subjects with ADNC and various combinations of other neuropathologies were consistently given primary antemortem clinical diagnoses consistent with AD, regardless of which additional pathologies were also present, indicating that ADNC has a stronger phenotype than other pathologies. Interestingly, subjects with concomitant LATE-NC and ADNC as well as subjects with 3-4 concurrent pathologies were given primary clinical diagnoses of AD in approximately 80%-92% of subjects, actually higher than the rate of AD diagnoses in subjects that had isolated ADNC (Figure 3C).
Figure 3.
Clinical associations with isolated and combined common neurodegenerative pathologies. (A) Venn diagram demonstrating the degree of overlap between subjects with autopsy-confirmed Alzheimer disease neuropathologic change (ADNC), limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC), Lewy body disease (LBD), and cerebrovascular disease (CVD). (B) Heatmap demonstrating the ADNC level, LATE-NC stage, LBD stage, number of present cerebrovascular variables (infarct, hemorrhage, moderate–severe arteriolosclerosis, and moderate–severe white matter rarefaction), and global Clinical Dementia Rating (CDR) across the cohort with isolated and combined neurodegenerative pathologies. (C) Primary diagnoses in subjects with various combinations of autopsy-confirmed ADNC, LATE-NC, LBD, and CVD. (D) Pairwise correlation between clinical phenotypes and various combinations of autopsy-confirmed neuropathology, calculated using Phi correlation coefficients. (E) Pairwise correlation between psychiatric/behavioral symptoms and various combinations of autopsy-confirmed neuropathology, calculated using Phi correlation coefficients. *P < .05; **P < .01; ***P < .001; ****P < .0001. The primary diagnosis Other* consists of additional underlying causes for cognitive impairment, detailed in Table S2. Abbreviations: bvFTD, behavioral variant frontotemporal dementia; PCA, posterior cortical atrophy; PPA, primary progressive aphasia.
In terms of clinical phenotypes, isolated ADNC and isolated LBD demonstrated distinct clinical features, whereas isolated LATE-NC demonstrated features similar to ADNC but with a lesser degree of impairment in most measures (Figure 3D). Psychiatric/behavioral symptoms, however, appeared much more varied (Figure 3E). Subjects with ADNC and comorbid LATE-NC demonstrated a similar pattern of cognitive impairment but to a greater degree compared to subjects with isolated ADNC, suggesting an additive or synergistic effect of these 2 pathologies. The clinical phenotype associated with LBD was only pronounced in subjects without comorbid ADNC; the distinct LBDS and Parkinsonism found in subjects with isolated LBD and LBD with CVD were significantly less prominent in subjects with concomitant intermediate- or high-level ADNC, a feature of these 2 comorbid disorders that has been described previously with increasing Braak stages.9 Notably, concurrent LATE-NC and LBD were absent in this cohort and concurrent LATE-NC, LBD, and CVD was a very rare combination (n = 2), thus making it impossible to draw meaningful conclusions regarding these clinical phenotypes based on these data.
Clinicopathologic correlations of ADNC mixed with FTLD-TDP, PSP, and CBD
To evaluate the clinical effects of concurrent ADNC and various forms of FTLD, we identified 6 cases with ADNC and FTLD-TDP, 15 cases with ADNC and PSP, and 7 cases with ADNC and CBD. Only a single case with both ADNC and PiD met the inclusion criteria, so correlations were not calculated for those combined pathologies. Unlike interactions between ADNC and LATE-NC/LBD/CVD, the primary clinical diagnoses (Figure 4A–C) and clinical phenotypes (Figure 4D–F) of concurrent ADNC and FTLD-TDP, PSP, and CBD were more significantly influenced by the presence of both pathologies. The combinations of these more phenotypically “dominant” pathologies resulted in more generalized cognitive impairment rather than the selective impairment characteristic of each pathology. For example, isolated ADNC was strongly correlated with amnestic dementia (while FTLD-TDP was not); isolated FTLD-TDP was correlated with semantic variant PPA and bvFTD (while ADNC was not); and the combined ADNC/FTLD-TDP cohort featured correlations with each of these pathologies that were generally milder than the isolated pathologies alone (Figure 4D). Similar trends were found in PSP (Figure 4E) and CBD (Figure 4F).
Figure 4.
Clinical associations with Alzheimer disease neuropathologic change (ADNC) and other TDP-43 and 4R tau proteinopathies. Primary diagnoses in subjects with combinations of ADNC and frontotemporal lobar dementia with TDP-43 (FTLD-TDP) (A), progressive supranuclear palsy (PSP) (B), and corticobasal degeneration (CBD) (C), as well as pairwise correlation between clinical phenotypes in subjects with combinations of ADNC and FTLD-TDP (D), PSP (E), and CBD (F). *P < .05; **P < .01; ***P < .001; ****P < .0001. The primary diagnosis Other* consists of additional underlying causes for cognitive impairment, detailed in Table S2. Abbreviations: bvFTD, behavioral variant frontotemporal dementia; PCA, posterior cortical atrophy; PPA, primary progressive aphasia.
Multivariate analysis of the clinical impact of comorbid neuropathologies
Multivariate logistic regression analysis was conducted to extract the effect of each individual underlying neuropathologic process in the full cohort with multiple mixed pathologies (n = 4624). The results were largely consistent with the cognitive effects of isolated pathologies. Alzheimer disease neuropathologic change was independently associated with amnestic dementia, logopenic PPA, and impairments in memory, orientation, judgment, language, visuospatial function, and attention, and was inversely correlated with Parkinsonism and PSP syndrome (Figure 5A). LATE-NC demonstrated an independent association with amnestic dementia, as well as impairments in memory, orientation, judgment, language, and attention (Figure 5B). Lewy body disease exhibited a strong correlation with LBDS and Parkinsonism (as expected), as well as amnestic dementia and additional impairments in orientation, judgment, language, visuospatial function, and attention (Figure 5C). Frontotemporal lobar degeneration with TDP-43, PiD, PSP, and CBD were generally more strongly associated with various subtypes of PPA and bvFTD, as well as varied impairments in memory, orientation, judgment, language, visuospatial function, and attention (Figure 5D–G). Cerebrovascular disease demonstrated focal and relatively mild independent effects on various cognitive domains, while AGD was not associated with any specific impairment (Figure 5H and I).
Figure 5.
Multivariate logistic regression analysis between clinical phenotypes and autopsy-confirmed Alzheimer disease neuropathologic change (ADNC) (A), limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) (B), Lewy body disease (LBD) (C), frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) (D), Pick disease (PiD) (E), progressive supranuclear palsy (PSP) (F), corticobasal degeneration (CBD) (G), argyrophilic grain disease (AGD) (H), and cerebrovascular disease (CVD) (I). Significance and odds ratios for each cognitive/neuropsychological test were determined in the context of a multivariate model including ADNC, primary age-related tauopathy (PART), LATE-NC, LBD, FTLD-TDP, amyotrophic lateral sclerosis (ALS), PiD, PSP, CBD, AGD, ARTAG, CVD, and hippocampal sclerosis, with ADRC, year of final clinical evaluation, age, sex, and years of education used as covariates. Abbreviations: ADRC, Alzheimer’s Disease Research Center; bvFTD, behavioral variant frontotemporal dementia; PPA, primary progressive aphasia.
To increase the granularity of these clinicopathologic correlations, multivariate logistic regression analysis was conducted to evaluate the effects of increasing ADNC levels,52 LATE-NC stages,61 LBD stages,58 and various forms of CVD. Similar to the presence/absence of intermediate/high levels of ADNC, progressive ADNC levels were associated with amnestic dementia and logopenic PPA, as well as impairment of memory, orientation, judgment, language, visuospatial function, and attention (Figure S1A). Progressive LATE-NC stages were associated with amnestic dementia and impairment in memory, orientation, judgment, language, and attention (Figure S1B), while progressive LBD stages are associated with a clinical LBDS, Parkinsonism, amnestic dementia, and impairment in orientation, judgment, language, attention, and visuospatial function (Figure S1C). Memory was independently impaired by the presence of infarcts and moderate–severe arteriolosclerosis, attention was impaired by the presence of infarcts and moderate–severe white matter rarefaction, judgment was impaired by infarcts, and the language and visuospatial domains were impaired by the presence of moderate–severe white matter rarefaction. Interestingly, the presence of moderate–severe arteriolosclerosis was also statistically associated with Parkinsonism, agitation/aggression, and irritability/lability (Figure S2).
In terms of psychiatric and behavioral symptoms, ADNC was independently associated with the presence of delusions, hallucinations, apathy, disinhibition, anxiety, agitation/aggression, irritability/lability, and disturbance in motor function (Figure 6A). LATE-NC demonstrated independent associations with only apathy and disinhibition (Figure 6B). Lewy body disease exhibited independent correlations with hallucinations, as well as disturbances in motor function and nighttime behavior (Figure 6C). Frontotemporal lobar degeneration with TDP-43 was independently associated with apathy, disinhibition, and appetite disturbance (Figure 6D), while PiD was only associated with disinhibition (Figure 6E). Progressive supranuclear palsy was associated with apathy (Figure 6F) and CBD was associated with disturbances in motor function and nighttime behavior (Figure 6G). Argyrophilic grain disease was not associated with any specific psychiatric symptom (Figure 6H). Cerebrovascular disease was independently associated with apathy, agitation/aggression, irritability/lability, and disturbance of appetite (Figure 6I). Aging-related tau astrogliopathy had no definitive deleterious effect on any evaluated clinical phenotype or symptom (Figure S3).
Figure 6.
Multivariate logistic regression analysis between psychiatric/behavioral symptoms and autopsy-confirmed Alzheimer disease neuropathologic change (ADNC) (A), limbic-predominant age-related TDP-43 encephalopathy neuropathologic change (LATE-NC) (B), Lewy body disease (LBD) (C), frontotemporal lobar degeneration with TDP-43 (FTLD-TDP) (D), Pick disease (PiD) (E), progressive supranuclear palsy (PSP) (F), corticobasal degeneration (CBD) (G), argyrophilic grain disease (AGD) (H), and cerebrovascular disease (CVD) (I). Significance and odds ratios for each cognitive/neuropsychological test were determined in the context of a multivariate model including ADNC, primary age-related tauopathy (PART), LATE-NC, LBD, FTLD-TDP, amyotrophic lateral sclerosis (ALS), PiD, PSP, CBD, AGD, ARTAG, CVD, and hippocampal sclerosis, with ADRC, year of final clinical evaluation, age, sex, and years of education used as covariates. Abbreviation: ADRC, Alzheimer’s Disease Research Center.
DISCUSSION
Dementia and its associated morbidity and mortality are a growing worldwide health threat, with a prevalence estimated to approximately triple over the next 3 decades as the global population ages. While ADNC is the most common underlying cause of dementia,2,4 the prevalence of clinical dementia resulting from multiple concurrent pathologies is being increasingly recognized, yet our understanding of the collective impact of mixed pathologies and the specific contributions of each proteinopathy to the overall clinical profile of individual subjects remains relatively limited.7,10,13,14,16,17,63 Additionally, identification of subjects who are cognitively “resilient” in the face of documented neurodegenerative pathology further complicates this picture, and a degree of the apparent resilience may be explained by the absence of certain copathologies, and thus may more accurately represent resistance to comorbid pathologies.64–70 Investigation of less common neurodegenerative pathologies is also hindered by the relative ubiquity of some level of ADNC in the elderly population that may alter the clinical profile of these other pathologies in subtle or overt ways.9,71 This overlap of neurodegenerative pathologies, at both the individual and population level, has hindered the development of useful clinical biomarkers and treatments based on each proteinopathy, delaying fundamental solutions to this growing social burden.
In this study, we further explored the effects of isolated and mixed pathologies on cognitive function, with a focus on their clinical presentations in a cohort of 1051 cases with select isolated and mixed pathology as well as 4624 cases for multivariate models.21 Our analyses of subjects with isolated and mixed pathologies consistently suggested several key findings. Several of the neurodegenerative pathologies investigated have well-defined clinical features (Table 2). As an isolated pathology and in multivariate logistic regression models, ADNC was relatively consistently given a primary clinical diagnosis of AD and had a well-defined amnestic dementia phenotype as well as a number of psychiatric features. Frontotemporal lobar degeneration with TDP-43 and PiD were more defined by disinhibition, bvFTD, and variants of PPA (while lacking an association with amnestic dementia). Lewy body disease was defined by Lewy body syndrome (including a strong association with hallucinations) and Parkinsonism (Figures 2, 5, and 6).9,20,58,62,71–75 Subjects with LATE-NC resembled a milder and more restricted version of ADNC, although only ADNC was associated with logopenic PPA and impairment in visuospatial function. In addition, while both ADNC and LATE-NC were independently associated with apathy and disinhibition, ADNC was additionally associated with delusions, hallucinations, anxiety, agitation/aggression, irritability/lability and disturbance in motor function. Previous studies have indicated that while both ADNC and LATE-NC primarily affect cognitive tests associated with logical memory (as well as Boston naming), ADNC affects digit span, trail-making, digit substitution, and animal/vegetable naming tests, while LATE-NC does not, thereby suggesting symptoms and tests that could potentially be used to distinguish these pathologies clinically (Figures 5 and 6; Table 2).8,21,28,29,31,61,76,77 Primary age-related tauopathy was not independently associated with significant impairment of cognitive domains or a specific clinical phenotype,36,37,39,55,56,78,79 but it is possible that current clinical and neurocognitive testing simply does not adequately assess mild or very specific clinical features that may be present in these disorders that appear to lack distinct clinical features. Emerging machine learning may help to further cluster specific deficits into groups to better define the clinical consequences of these pathologies and aid in future antemortem diagnosis.
Table 2.
Summary of the significant independent effects of each neurodegenerative pathology.
| Neuropathology | Clinical phenotypes | Cognitive domains impaired | Psychiatric/Behavioral features | Neurocognitive testing a |
|---|---|---|---|---|
| ADNC | Amnestic dementia | Memory | Delusionsb | Logical Memory, Immediate |
| Logopenic PPAb | Orientation | Hallucinations | Logical Memory, Delayed | |
| Judgment | Apathy | Digit Span Forward | ||
| Language | Disinhibition | Digit Span Backward | ||
| Visuospatial | Anxietyb | Trail Making Test, Part A | ||
| Attention | Agitation/Aggression | Trail Making Test, Part B | ||
| Irritability/Lability | WAIS Digit Substitution | |||
| Motor function | Animal Naming | |||
| Vegetable Naming | ||||
| Boston Naming Test | ||||
| LATE-NC | Amnestic dementia | Memory | Apathy | Logical Memory, Immediate |
| Orientation | Disinhibition | Logical Memory, Delayed | ||
| Judgment | Boston Naming Test | |||
| Language | ||||
| Attention | ||||
| LBD | Amnestic dementia | Orientation | Hallucinations | Digit Span Backward |
| Lewy body syndromeb | Judgment | Nighttime behavior | Trail Making Test, Part A | |
| Parkinsonismb | Language | Motor function | Vegetable Naming | |
| Visuospatial | ||||
| Attention | ||||
| FTLD-TDP | Semantic PPA | Memory | Apathy | Logical Memory, Immediate |
| bvFTD | Orientation | Disinhibition | Logical Memory, Delayed | |
| Judgment | Appetite disturbance | Digit Span Forward | ||
| Language | Digit Span Backward | |||
| Visuospatial | WAIS Digit Substitution | |||
| Attention | Animal Naming | |||
| Vegetable Naming | ||||
| Boston Naming Test | ||||
| PiD | Semantic PPA | Memory | Disinhibition | Logical Memory, Immediate |
| Nonfluent/Agrammatic PPA | Judgment | Logical Memory, Delayed | ||
| bvFTD | Language | Digit Span Backward | ||
| Visuospatial | Animal Naming | |||
| Attention | Vegetable Naming | |||
| PSP | Nonfluent/agrammatic PPA | Orientation | Apathy | Trail Making Test, Part A |
| PSP syndromeb | Judgment | Animal Naming | ||
| CBD syndrome | Language | |||
| CBD | Nonfluent/agrammatic PPA | Judgment | Nighttime behavior | Logical Memory, Immediate |
| bvFTD | Language | Motor function | Logical Memory, Delayed | |
| CBD syndrome | Visuospatial | Digit Span Forward | ||
| Trail Making Test, Part A | ||||
| Boston Naming Test | ||||
| CVD | — | Memory | Apathy | Trail Making Test, Part A |
| Judgment | Agitation/Aggression | Trail Making Test, Part B | ||
| Irritability/Lability | ||||
| Appetite disturbance |
Abbreviations: ADNC, Alzheimer disease neuropathologic change; AGD, argyrophilic grain disease; ALS, amyotrophic lateral sclerosis; CBD, corticobasal degeneration; CVD, cerebrovascular disease; FTLD-TDP, frontotemporal lobar degeneration with TDP-43; LATE-NC, limbic-predominant age-related TDP-43 encephalopathy neuropathologic change; LBD, Lewy body disease; PiD, Pick disease; PSP, progressive supranuclear palsy.
Significantly impaired neurocognitive testing derived from data analyzed in Maldonado-Diaz et al. (21).
Clinical feature that is unique to a particular neurodegenerative pathology in this dataset based on multivariate linear regression analysis.
In relation to LATE-NC, LBD, and CVD, ADNC appears to represent a more “dominant” pathology with respect to an individual’s clinical phenotype (Figure 3). When various combinations of these pathologies are concurrent with ADNC, the clinical presentation is far more similar to that of ADNC than to the clinical features found in these other isolated disorders. In the cohorts with comorbid ADNC and LATE-NC, subjects were given a primary clinical diagnosis of AD more commonly than subjects with isolated ADNC (Figure 3C), and the clinical features and cognitive testing appeared more exaggerated toward an “ADNC-like” pattern (Figure 3D). Conversely, the more distinctive clinical features of LBD appeared to be somewhat obscured by the presence of ADNC (and less so by LATE-NC and CVD), while the clinical features of ADNC remained intact. These trends became more prominent in subjects with additional comorbid pathologies. Conversely, FTLD-TDP, PiD, PSP, and CBD also appeared to have more “dominant” clinical phenotypes. Unlike subjects with comorbid LATE-NC, LBD, or CVD, subjects with these pathologies and comorbid ADNC have a more mixed clinical phenotype with less association with amnestic dementia, and more correlation with PPA variants, bvFTD, PSP syndrome, and CBD syndrome (Figure 4). It is also possible that clinical and cognitive testing have simply been better tailored to AD (and these frontotemporal dementias), and so are better able to identify features of these pathologies. Clinically, “probable AD” is a relatively nonspecific phenotype characterized by a wide-ranging global cognitive impairment (which includes amnestic dementia as a component), some of which may be independently produced by other pathologies, including LATE-NC (Table 2); a subset of these cases without ADNC at autopsy was given antemortem clinical diagnoses of AD (Figure 2A). Lewy body disease is an historically well-characterized disorder with distinctive clinical features but these distinct features still appeared to be relatively muted in the presence of ADNC (Figure 3D); this finding was previously identified in the interaction of ADNC and LBD.9
Given the cognitive effects of these combined pathologies and recent studies that estimate the degree of cognitive impairment that could hypothetically be removed from a population with a “magic bullet” therapy to remove a specific pathologic process,21,80,81 this becomes an even more important consideration. For example, if a therapeutic could be developed to fully remove ADNC, the portion of the population with concurrent ADNC and LATE-NC may still retain a significant degree of “AD-like” manifestations caused by underlying LATE-NC, including impaired global cognition, impaired memory, orientation, judgment, and visuospatial function, and an amnestic dementia phenotype (Figure 3). Additionally, subjects with isolated LATE-NC have a mean global CDR of 1.6 ± 0.3, statistically equivalent to subjects with concomitant ADNC and LATE-NC (2.0 ± 0.2; P = .2838). This principle will make the development of antemortem biomarkers to identify non-ADNC comorbid conditions (and their extent) increasingly important as personalized medicine begins to develop therapies for these individual disorders.
Previously it was demonstrated that the presence of APOE ε4 alleles was significantly associated with the accumulation of multiple neurodegenerative proteinopathies.12,15,21 Here, it appears that this effect is driven primarily by the presence of ADNC, that is, there was no significant increase in the frequency of APOE ε4 alleles in any other isolated neuropathology, although the addition of multiple other neurodegenerative pathologies, including LATE-NC and LBD, may be associated with increased frequency of APOE ε4 alleles in combination with ADNC (Table 1).
There are several limitations to this study. While the cohort used was large, most categories of isolated neuropathologies are relatively small (Table 1), and all data were obtained from NACC, which may not be representative of the general population.23,82 Enrollment of subjects in AD research centers and the NACC cohort generally prioritizes patients with memory and cognitive symptoms, in particular the AD clinical phenotype, with lower rates of inclusion of patients with primary behavioral or motor symptoms. This cohort is also highly enriched for individuals with APOE ε2 alleles (∼44% of the overall cohort).21 This cohort also consists of subjects evaluated at multiple different ADRC sites (Table S1) over an extended period of time and may include regional differences in dementia diagnoses.83 To address this limitation, we used multivariate logistic regression analysis to evaluate these clinicopathologic correlations in a larger cohort with various combinations of neuropathological conditions, adjusting for ADRC sites among other variables. While scores of cognitive testing may be relatively objective and reproducible between different settings, the clinical diagnoses and other assessments were performed by many individuals working in different clinical eras (both as individual and consensus assessments), and advancements in imaging and biomarker studies have been used in different institutions at different times. Clinical and neuropathologic diagnostic criteria have also changed over time and new disease entities, such as LATE-NC, which has only been officially defined since 2019, have been recognized; however, this diagnosis is frequently discernable from older recorded TDP-43 data.21,41,61 The effect of shifting global consensus on some entities on clinical decision-making and data collection is difficult to determine. Additionally, the neuropathologic data available generally assess the unilateral regionality of pathology and not asymmetry or local severity, which may be important in some diseases, particularly in the earlier stages.15,56,59,63,78,84–86 Other variables such as FTLD-TDP assessment and TDP-43 distribution do not take into account subtypes, density of the pathology, or detailed location of the pathology beyond a few binary variables. The distinction between LATE-NC and FTLD-TDP remains a controversial issue61,87–89; FTLD-TDP types A and B appear to overlap with LATE-NC stage 3, and there are a number of more recent recommendations to distinguish between these 2 disease processes.60,77,90 Given this limitation, subjects included in this study as either LATE-NC or FTLD-TDP from the available NACC data had significantly different frequencies of hippocampal sclerosis, participant ages, primary diagnoses/clinical features, and interactions with ADNC, indicating that these are in fact separate entities, and that our classification was successful in separating most representative cases from each entity.
Despite some limitations, these results corroborate the known cumulative effects of mixed pathologies, further disentangle a variety of interactions between each pathology that contribute to a variety of clinical dementia presentations, and partially support the conceptualization of new dementia nomenclature separating clinical presentation from the underlying pathology.91 The rational development of disease-modifying therapies and preventative agents requires precise understanding of which pathologies are present in a given individual or population, and developing biomarkers for definitive antemortem diagnoses of individual neuropathological processes requires parallel efforts to understand the interactions between different neuropathologies and the resulting complex cognitive and behavioral phenotypes. The present study represents a significant step forward in understanding the clinical phenotypes associated with specific neurodegenerative pathologic processes and addressing the complex interplay between these disorders.
Supplementary Material
Acknowledgments
The authors would like to thank Henne Holstege and Jeroen Hoozemans for their critical review of this manuscript.
Contributor Information
Satomi Hiya, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
Carolina Maldonado-Díaz, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
Susan K Rohde, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Department of Pathology, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Neuroscience, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Human Genetics, Genomics of Neurodegenerative Diseases and Aging, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; Department of Neurology, Alzheimer Center Amsterdam, Neuroscience, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Mitzi M Gonzales, Department of Neurology, Cedars Sinai Medical Center, Los Angeles, CA, United States; Department of Neurology, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States; Glenn Biggs Institute for Alzheimer’s & Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States.
Leyla Canbeldek, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
Lakshmi S Kulumani Mahadevan, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
Raquel T Yokoda, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
A Campbell Sullivan, Department of Neurology, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States; Glenn Biggs Institute for Alzheimer’s & Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States.
Alicia S Parker, Department of Neurology, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States; Glenn Biggs Institute for Alzheimer’s & Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States.
Charles L White, III, Department of Pathology, University of Texas Southwestern Medical Center, Dallas, TX, United States.
Elena V Daoud, Department of Pathology, University of Texas Southwestern Medical Center, Dallas, TX, United States.
Victoria Flores-Almazan, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Neuropathology Brain Bank & Research CoRE, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
John F Crary, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Neuropathology Brain Bank & Research CoRE, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Department of Artificial Intelligence & Human Health, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kurt Farrell, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Neuropathology Brain Bank & Research CoRE, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Department of Artificial Intelligence & Human Health, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Ronald M. Loeb Center for Alzheimer’s Disease, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jamie M Walker, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Glenn Biggs Institute for Alzheimer’s & Neurodegenerative Diseases, University of Texas Health Science Center at San Antonio, San Antonio, TX, United States; Neuropathology Brain Bank & Research CoRE, Icahn School of Medicine at Mount Sinai, New York, NY, United States; Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
Timothy E Richardson, Department of Pathology, Molecular and Cell-Based Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, United States.
Supplementary Material
Supplementary material is available at academic.oup.com/jnen.
Funding
J.M.W. and T.E.R. are supported in part by National Institute on Aging (NIA) R21 AG078505 and Texas Alzheimer’s Research and Care Consortium (TARCC) grants 957581 and 957607. E.V.D. is also supported in part by TARCC grant 957607. M.M.G. is supported in part by NIA R01 AG077472. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The NACC database is funded by NIA/NIH grant U24 AG072122. The NACC data are contributed by the NIA-funded ADRCs: P30 AG062429 (PI James Brewer, MD, PhD), P30 AG066468 (PI Oscar Lopez, MD), P30 AG062421 (PI Bradley Hyman, MD, PhD), P30 AG066509 (PI Thomas Grabowski, MD), P30 AG066514 (PI Mary Sano, PhD), P30 AG066530 (PI Helena Chui, MD), P30 AG066507 (PI Marilyn Albert, PhD), P30 AG066444 (PI John Morris, MD), P30 AG066518 (PI Jeffrey Kaye, MD), P30 AG066512 (PI Thomas Wisniewski, MD), P30 AG066462 (PI Scott Small, MD), P30 AG072979 (PI David Wolk, MD), P30 AG072972 (PI Charles DeCarli, MD), P30 AG072976 (PI Andrew Saykin, PsyD), P30 AG072975 (PI David Bennett, MD), P30 AG072978 (PI Neil Kowall, MD), P30 AG072977 (PI Robert Vassar, PhD), P30 AG066519 (PI Frank LaFerla, PhD), P30 AG062677 (PI Ronald Petersen, MD, PhD), P30 AG079280 (PI Eric Reiman, MD), P30 AG062422 (PI Gil Rabinovici, MD), P30 AG066511 (PI Allan Levey, MD, PhD), P30 AG072946 (PI Linda Van Eldik, PhD), P30 AG062715 (PI Sanjay Asthana, MD, FRCP), P30 AG072973 (PI Russell Swerdlow, MD), P30 AG066506 (PI Todd Golde, MD, PhD), P30 AG066508 (PI Stephen Strittmatter, MD, PhD), P30 AG066515 (PI Victor Henderson, MD, MS), P30 AG072947 (PI Suzanne Craft, PhD), P30 AG072931 (PI Henry Paulson, MD, PhD), P30 AG066546 (PI Sudha Seshadri, MD), P20 AG068024 (PI Erik Roberson, MD, PhD), P20 AG068053 (PI Justin Miller, PhD), P20 AG068077 (PI Gary Rosenberg, MD), P20 AG068082 (PI Angela Jefferson, PhD), P30 AG072958 (PI Heather Whitson, MD), P30 AG072959 (PI James Leverenz, MD).
Conflicts of Interest
TER is an editorial board member of this journal, but was not involved in the peer-review process nor had access to any information regarding its peer review. The other authors declare that they have no competing interests, conflicts of interest, or other relevant disclosures. The results presented in this paper have not been published previously in whole or in part.
Data Availability
The data presented in this manuscript are derived from the National Alzheimer’s Coordinating Center (NACC) dataset and are available upon request from https://naccdata.org/.
References
- 1. Collaborators GBDDF. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7:e105-e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prince M, Bryce R, Albanese E, et al. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63-75.e2. [DOI] [PubMed] [Google Scholar]
- 3. Wimo A, Seeher K, Cataldi R, et al. The worldwide costs of dementia in 2019. Alzheimers Dement. 2023;19:2865-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scambray KA, Nguyen HL, Sajjadi SA. Association of vascular and degenerative brain pathologies and past medical history from the National Alzheimer’s Coordinating Center Database. J Neuropathol Exp Neurol. 2023;82:390-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jack CR Jr, Therneau TM, Weigand SD, et al. Prevalence of biologically vs clinically defined Alzheimer spectrum entities using the National Institute on Aging-Alzheimer’s Association Research Framework. JAMA Neurol. 2019;76:1174-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beach TG, Malek-Ahmadi M. Alzheimer’s disease neuropathological comorbidities are common in the younger-old. J Alzheimers Dis. 2021;79:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forrest SL, Kovacs GG. Current concepts of mixed pathologies in neurodegenerative diseases. Can J Neurol Sci. 2023;50:329-345. [DOI] [PubMed] [Google Scholar]
- 8. Hiya S, Maldonado-Diaz C, Walker JM, et al. Cognitive symptoms progress with limbic-predominant age-related TDP-43 encephalopathy stage and co-occurrence with Alzheimer disease. J Neuropathol Exp Neurol. 2023;83:2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nichols E, Merrick R, Hay SI, et al. The prevalence, correlation, and co-occurrence of neuropathology in old age: harmonisation of 12 measures across six community-based autopsy studies of dementia. Lancet Healthy Longev. 2023;4:e115-e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabinovici GD, Carrillo MC, Forman M, et al. Multiple comorbid neuropathologies in the setting of Alzheimer’s disease neuropathology and implications for drug development. Alzheimers Dement (N Y). 2017;3:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141:2181-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robinson JL, Richardson H, Xie SX, et al. The development and convergence of co-pathologies in Alzheimer’s disease. Brain. 2021;144:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tome SO, Thal DR. Co-pathologies in Alzheimer’s disease: just multiple pathologies or partners in crime? Brain. 2021;144:706-708. [DOI] [PubMed] [Google Scholar]
- 15. Walker JM, Richardson TE. Cognitive resistance to and resilience against multiple comorbid neurodegenerative pathologies and the impact of APOE status. J Neuropathol Exp Neurol. 2023;82:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alafuzoff I, Libard S. Ageing-related neurodegeneration and cognitive decline. Int J Mol Sci. 2024;25:4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chatterjee A, Hirsch-Reinshagen V, Moussavi SA, et al. Clinico-pathological comparison of patients with autopsy-confirmed Alzheimer’s disease, dementia with Lewy bodies, and mixed pathology. Alzheimers Dement (Amst). 2021;13:e12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beach TG, Monsell SE, Phillips LE, et al. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J Neuropathol Exp Neurol. 2012;71:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cummings J, Lanctot K, Grossberg G, et al. Progress in pharmacologic management of neuropsychiatric syndromes in neurodegenerative disorders: a review. JAMA Neurol. 2024;81:645-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quadalti C, Palmqvist S, Hall S, et al. Clinical effects of Lewy body pathology in cognitively impaired individuals. Nat Med. 2023;29:1964-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maldonado-Diaz C, Hiya S, Yokoda RT, et al. Disentangling and quantifying the relative cognitive impact of concurrent mixed neurodegenerative pathologies. Acta Neuropathol. 2024;147:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gauthreaux K, Bonnett TA, Besser LM, et al. Concordance of clinical Alzheimer diagnosis and neuropathological features at autopsy. J Neuropathol Exp Neurol. 2020;79:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gauthreaux K, Kukull WA, Nelson KB, et al. Different cohort, disparate results: selection bias is a key factor in autopsy cohorts. Alzheimers Dement. 2024;20:266-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katsumata Y, Fardo DW, Kukull WA, et al. Dichotomous scoring of TDP-43 proteinopathy from specific brain regions in 27 academic research centers: associations with Alzheimer’s disease and cerebrovascular disease pathologies. Acta Neuropathol Commun. 2018;6:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sennik S, Schweizer TA, Fischer CE, et al. Risk factors and pathological substrates associated with agitation/aggression in Alzheimer’s disease: a preliminary study using NACC data. J Alzheimers Dis. 2017;55:1519-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Besser LM, Crary JF, Mock C, et al. Comparison of symptomatic and asymptomatic persons with primary age-related tauopathy. Neurology. 2017;89:1707-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Besser LM, Mock C, Teylan MA, et al. Differences in cognitive impairment in primary age-related tauopathy versus Alzheimer disease. J Neuropathol Exp Neurol. 2019;78:219-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Besser LM, Teylan MA, Nelson PT. Limbic predominant age-related TDP-43 encephalopathy (LATE): clinical and neuropathological associations. J Neuropathol Exp Neurol. 2020;79:305-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler Pagnotti RM, Pudumjee SB, Cross CL, et al. Cognitive and clinical characteristics of patients with limbic-predominant age-related TDP-43 encephalopathy. Neurology. 2023;100:e2027-e2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gauthreaux K, Mock C, Teylan MA, et al. Symptomatic profile and cognitive performance in autopsy-confirmed limbic-predominant age-related TDP-43 encephalopathy with comorbid Alzheimer disease. J Neuropathol Exp Neurol. 2022;81:975-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gauthreaux KM, Teylan MA, Katsumata Y, et al. Limbic-predominant age-related TDP-43 encephalopathy: medical and pathologic factors associated with comorbid hippocampal sclerosis. Neurology. 2022;98:e1422-e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hassenstab J, Monsell SE, Mock C, et al. Neuropsychological markers of cognitive decline in persons with Alzheimer disease neuropathology. J Neuropathol Exp Neurol. 2015;74:1086-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayden KM, Jones RN, Zimmer C, et al. Factor structure of the National Alzheimer’s Coordinating Centers uniform dataset neuropsychological battery: an evaluation of invariance between and within groups over time. Alzheimer Dis Assoc Disord. 2011;25:128-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nelson PT, Kryscio RJ, Jicha GA, et al. Relative preservation of MMSE scores in autopsy-proven dementia with Lewy bodies. Neurology. 2009;73:1127-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Serrano-Pozo A, Qian J, Muzikansky A, et al. Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the Alzheimer disease continuum. J Neuropathol Exp Neurol. 2016;75:516-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teylan M, Besser LM, Crary JF, et al. Clinical diagnoses among individuals with primary age-related tauopathy versus Alzheimer’s neuropathology. Lab Invest. 2019;99:1049-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Teylan M, Mock C, Gauthreaux K, et al. Cognitive trajectory in mild cognitive impairment due to primary age-related tauopathy. Brain. 2020;143:611-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Teylan MA, Mock C, Gauthreaux K, et al. Differences in symptomatic presentation and cognitive performance among participants with LATE-NC compared to FTLD-TDP. J Neuropathol Exp Neurol. 2021;80:1024-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walker JM, Gonzales MM, Goette W, et al. Cognitive and neuropsychological profiles in Alzheimer’s disease and primary age-related tauopathy and the influence of comorbid neuropathologies. J Alzheimers Dis. 2023;92:1037-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walker JM, Kazempour Dehkordi S, Schaffert J, et al. The spectrum of Alzheimer-type pathology in cognitively normal individuals. J Alzheimers Dis. 2023;91:683-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woodworth DC, Nguyen KM, Sordo L, et al. Comprehensive assessment of TDP-43 neuropathology data in the National Alzheimer’s Coordinating Center database. Acta Neuropathol. 2024;147:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Katsumata Y, Wu X, Aung KZ, et al. Pathologic correlates of aging-related tau astrogliopathy: ARTAG is associated with LATE-NC and cerebrovascular pathologies, but not with ADNC. Neurobiol Dis. 2024;191:106412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim J, Schweizer TA, Fischer CE, et al. Psychosis in “cognitively asymptomatic” elderly subjects is associated with neuritic plaque load, not neurofibrillary tangles. Alzheimer Dis Assoc Disord. 2018;32:185-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Devanand DP, Lee S, Huey ED, et al. Associations between neuropsychiatric symptoms and neuropathological diagnoses of Alzheimer disease and related dementias. JAMA Psychiatry. 2022;79:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liampas I, Siokas V, Lyketsos CG, et al. The relationship between neuropsychiatric symptoms and cognitive performance in older adults with normal cognition. Medicina (Kaunas). 2022;58:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sano M, Zhu CW, Neugroschl J, et al. Agitation in cognitive disorders: use of the National Alzheimer’s Coordinating Center Uniform Data Set (NACC-UDS) to evaluate International Psychogeriatric Association definition. Am J Geriatr Psychiatry. 2022;30:1198-1208. [DOI] [PubMed] [Google Scholar]
- 47. Guan DX, Rehman T, Nathan S, et al. Neuropsychiatric symptoms: risk factor or disease marker? A study of structural imaging biomarkers of Alzheimer’s disease and incident cognitive decline. Hum Brain Mapp. 2024;45:e70016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu CW, Schneider LS, Elder GA, et al. Neuropsychiatric symptom profile in Alzheimer’s disease and their relationship with functional decline. Am J Geriatr Psychiatry. 2024;32:1402-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Beekly DL, Ramos EM, van Belle G, et al. The National Alzheimer’s Coordinating Center (NACC) database: an Alzheimer disease database. Alzheimers Dis Assoc Disord. 2004;18:270-277. [PubMed] [Google Scholar]
- 50. Beekly DL, Ramos EM, Lee WW, et al. ; NIA Alzheimer’s Disease Centers. The National Alzheimer’s Coordinating Center (NACC) database: the uniform data set. Alzheimer Dis Assoc Disord. 2007;21:249-258. [DOI] [PubMed] [Google Scholar]
- 51. Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Montine TJ, Phelps CH, Beach TG, et al. ; Alzheimer’s Association. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239-259. [DOI] [PubMed] [Google Scholar]
- 54. Thal DR, Rub U, Orantes M, et al. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791-1800. [DOI] [PubMed] [Google Scholar]
- 55. Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. 2014;128:755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Walker JM, Richardson TE, Farrell K, et al. Early selective vulnerability of the CA2 hippocampal subfield in primary age-related tauopathy. J Neuropathol Exp Neurol. 2021;80:102-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Walker JM, Orr ME, Orr TC, et al. Spatial proteomics of hippocampal subfield-specific pathology in Alzheimer’s disease and primary age-related tauopathy. Alzheimers Dement. 2024;20:783-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McKeith IG, Dickson DW, Lowe J, et al. ; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863-1872. [DOI] [PubMed] [Google Scholar]
- 59. Attems J, Toledo JB, Walker L, et al. Neuropathological consensus criteria for the evaluation of Lewy pathology in post-mortem brains: a multi-centre study. Acta Neuropathol. 2021;141:159-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nelson PT, Lee EB, Cykowski MD, et al. LATE-NC staging in routine neuropathologic diagnosis: an update. Acta Neuropathol. 2023;145:159-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nelson PT, Dickson DW, Trojanowski JQ, et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report. Brain. 2019;142:1503-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang M, Ganz AB, Rohde S, et al. Resilience and resistance to the accumulation of amyloid plaques and neurofibrillary tangles in centenarians: an age-continuous perspective. Alzheimers Dement. 2023;19:2831-2841. [DOI] [PubMed] [Google Scholar]
- 64. Arenaza-Urquijo EM, Vemuri P. Resistance vs resilience to Alzheimer disease: clarifying terminology for preclinical studies. Neurology. 2018;90:695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. de Vries LE, Huitinga I, Kessels HW, et al. The concept of resilience to Alzheimer’s disease: current definitions and cellular and molecular mechanisms. Mol Neurodegener. 2024;19:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Montine KS, Berson E, Phongpreecha T, et al. Understanding the molecular basis of resilience to Alzheimer’s disease. Front Neurosci. 2023;17:1311157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Montine TJ, Cholerton BA, Corrada MM, et al. Concepts for brain aging: resistance, resilience, reserve, and compensation. Alzheimers Res Ther. 2019;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stern Y, Albert M, Barnes CA, et al. A framework for concepts of reserve and resilience in aging. Neurobiol Aging. 2023;124:100-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Walker JM, Kazempour Dehkordi S, Fracassi A, et al. Differential protein expression in the hippocampi of resilient individuals identified by digital spatial profiling. Acta Neuropathol Commun. 2022;10:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang M, Ganz AB, Rohde S, et al. The correlation between neuropathology levels and cognitive performance in centenarians. Alzheimers Dement. 2023;19:5036-5047. [DOI] [PubMed] [Google Scholar]
- 71. Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain. 2014;137:1176-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mesulam MM, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10:554-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kirshner HS. Frontotemporal dementia and primary progressive aphasia, a review. Neuropsychiatr Dis Treat. 2014;10:1045-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Giannini LAA, Xie SX, McMillan CT, et al. Divergent patterns of TDP-43 and tau pathologies in primary progressive aphasia. Ann Neurol. 2019;85:630-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu KY, Reeves S, McAleese KE, et al. Neuropsychiatric symptoms in limbic-predominant age-related TDP-43 encephalopathy and Alzheimer’s disease. Brain. 2020;143:3842-3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Robinson JL, Porta S, Garrett FG, et al. Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration. Brain. 2020;143:2844-2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Iida MA, Farrell K, Walker JM, et al. Predictors of cognitive impairment in primary age-related tauopathy: an autopsy study. Acta Neuropathol Commun. 2021;9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Savola S, Kaivola K, Raunio A, et al. Primary age-related tauopathy in a Finnish population-based study of the oldest old (Vantaa 85+). Neuropathol Appl Neurobiol. 2022;48:e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Boyle PA, Wang T, Yu L, et al. To what degree is late life cognitive decline driven by age-related neuropathologies? Brain. 2021;144:2166-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cholerton B, Latimer CS, Crane PK, et al. Neuropathologic burden and dementia in nonagenarians and centenarians. Neurology. 2024;102:e208060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Schneider JA, Aggarwal NT, Barnes L, et al. The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis. 2009;18:691-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bynum JPW, Benloucif S, Martindale J, et al. Regional variation in diagnostic intensity of dementia among older U.S. adults: an observational study. Alzheimers Dement. 2024;20:6755-6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Walker JM, Goette W, Farrell K, et al. ; PART Working Group. The relationship between hippocampal β-amyloid burden and spatial distribution of neurofibrillary degeneration. Alzheimers Dement. 2023;19:3158-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Marx GA, Koenigsberg DG, McKenzie AT, et al. ; PART Working Group. Artificial intelligence-derived neurofibrillary tangle burden is associated with antemortem cognitive impairment. Acta Neuropathol Commun. 2022;10:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Walker JM, Fudym Y, Farrell K, et al. Asymmetry of hippocampal tau pathology in primary age-related tauopathy and Alzheimer disease. J Neuropathol Exp Neurol. 2021;80:436-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Josephs KA, Mackenzie I, Frosch MP, et al. LATE to the PART-y. Brain. 2019;142:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nelson PT, Dickson DW, Trojanowski JQ, et al. Reply: LATE to the PART-y. Brain. 2019;142:e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Josephs KA, Murray ME, Tosakulwong N, et al. Pathological, imaging and genetic characteristics support the existence of distinct TDP-43 types in non-FTLD brains. Acta Neuropathol. 2019;137:227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Young AL, Vogel JW, Robinson JL, et al. Data-driven neuropathological staging and subtyping of TDP-43 proteinopathies. Brain. 2023;146:2975-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Petersen RC, Weintraub S, Sabbagh M, et al. ; Dementia Nomenclature Initiative. A new framework for dementia nomenclature. JAMA Neurol. 2023;80:1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this manuscript are derived from the National Alzheimer’s Coordinating Center (NACC) dataset and are available upon request from https://naccdata.org/.