Abstract
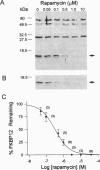
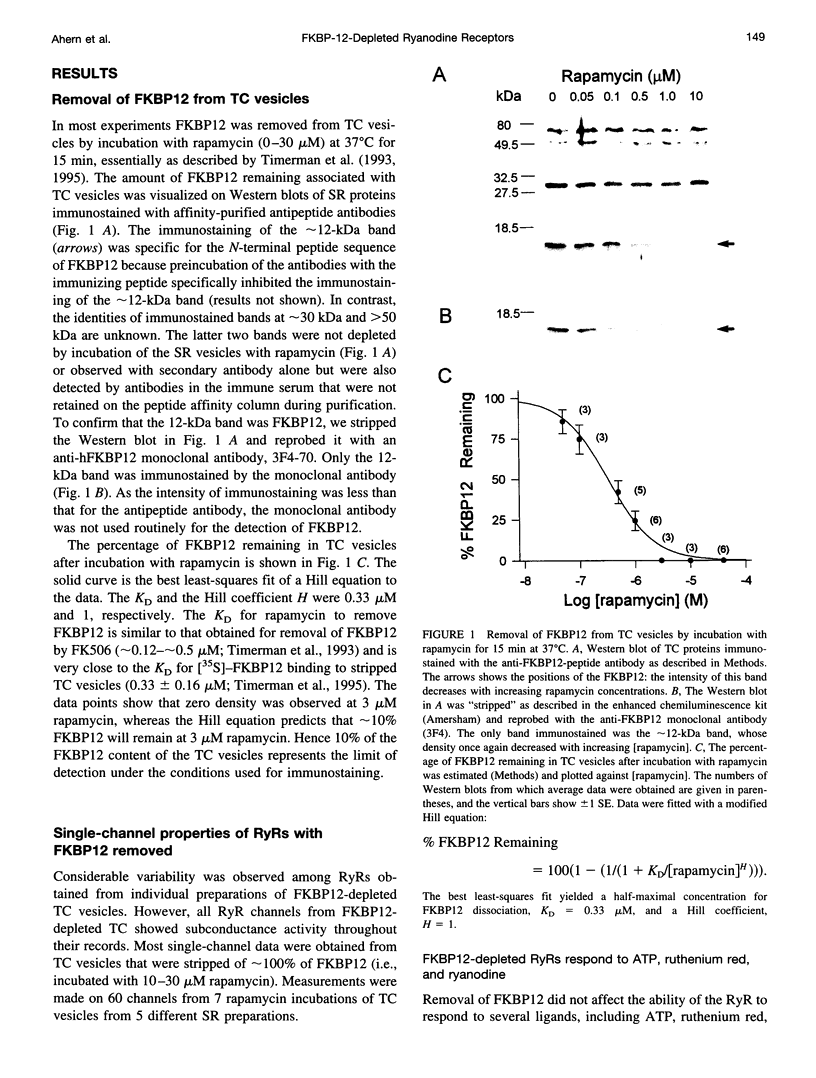
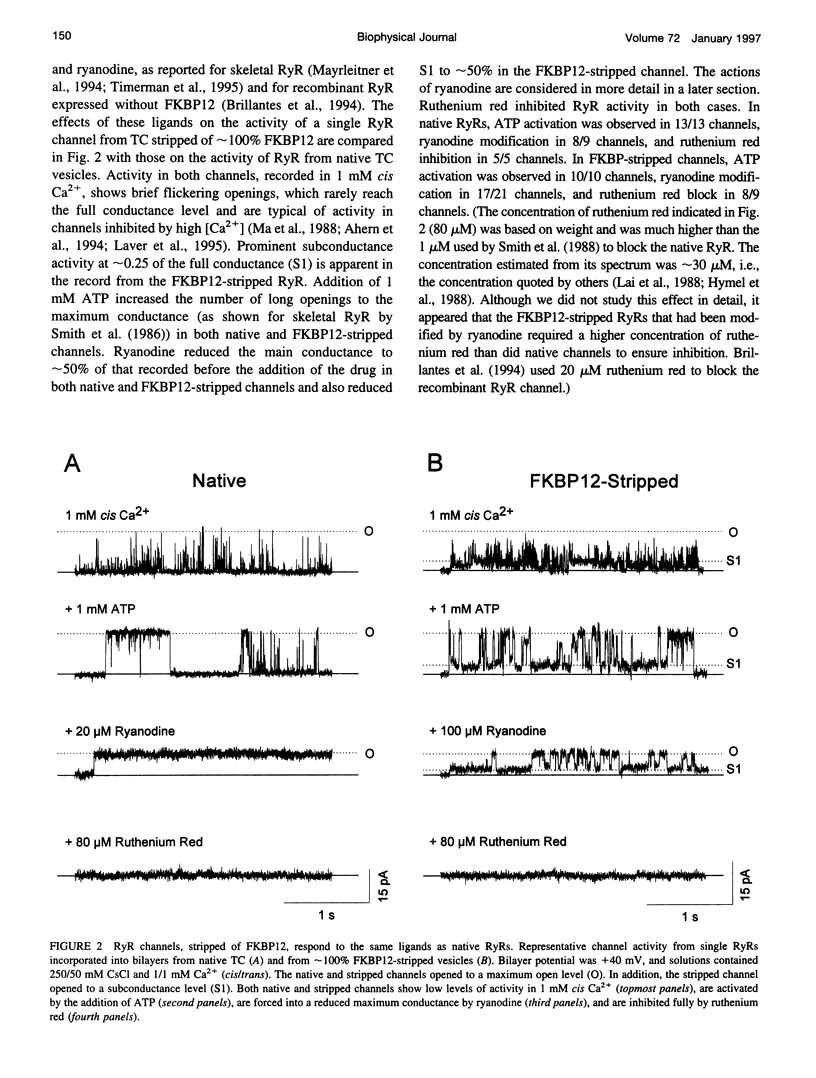
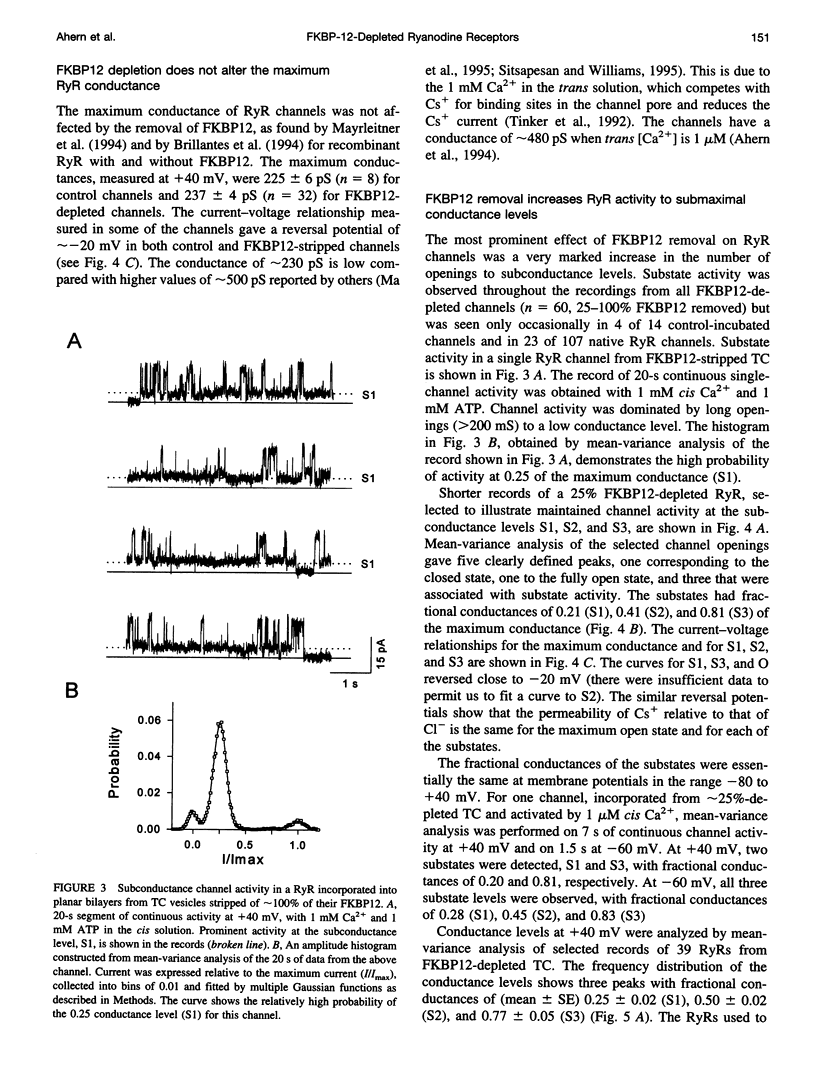
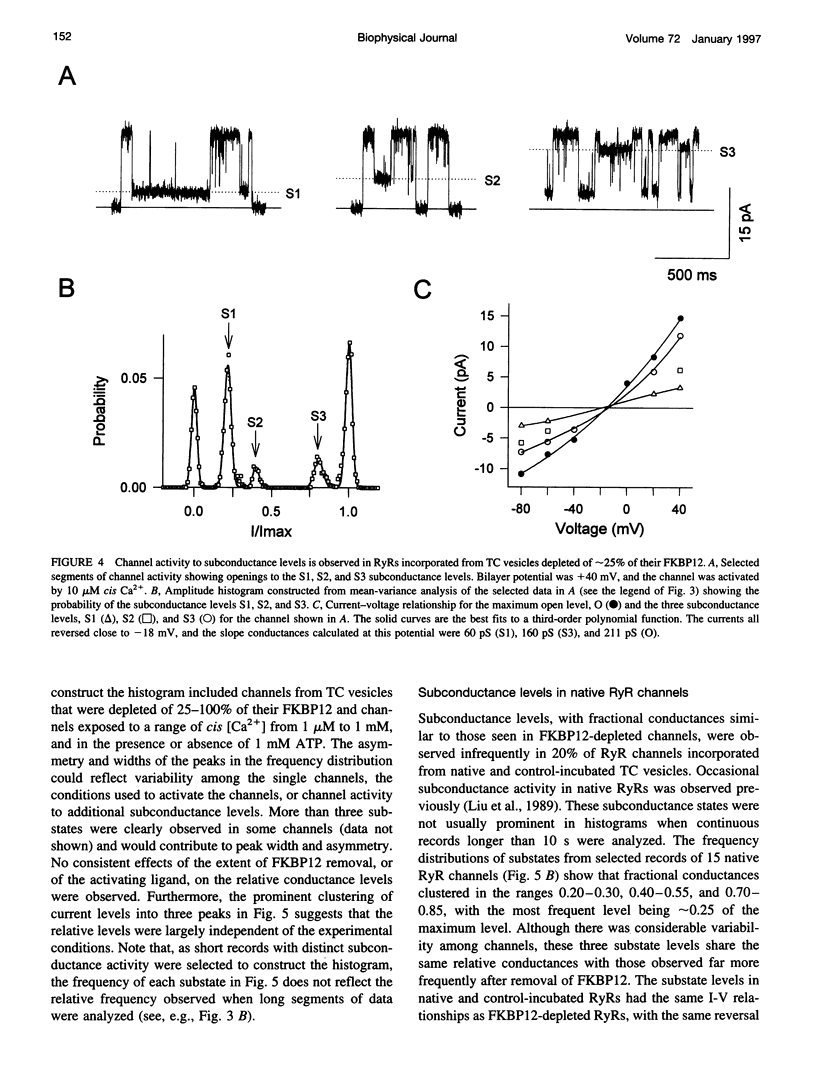
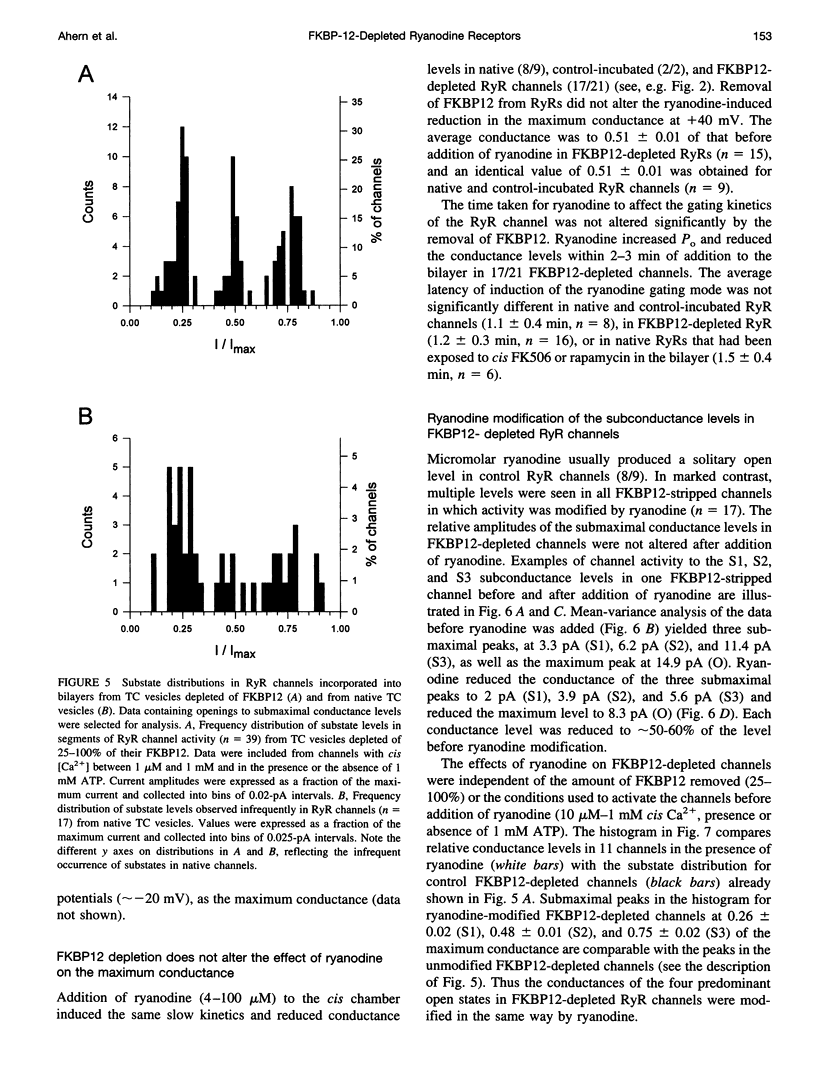
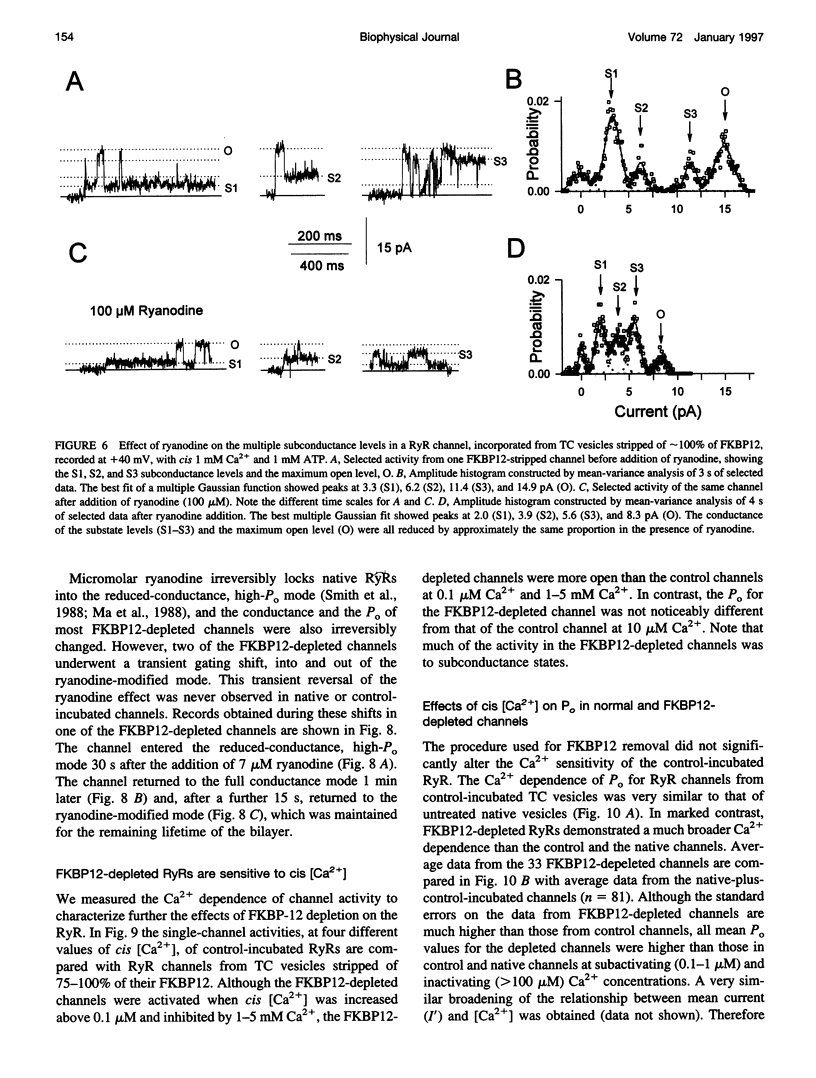
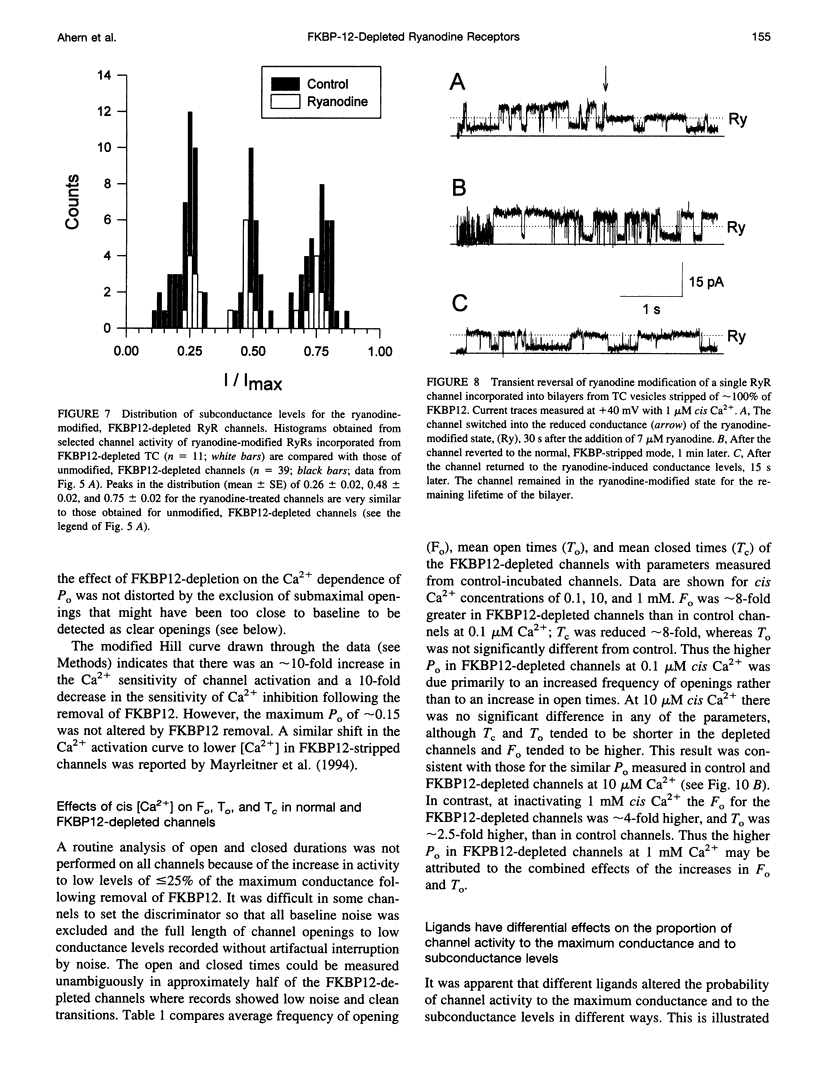
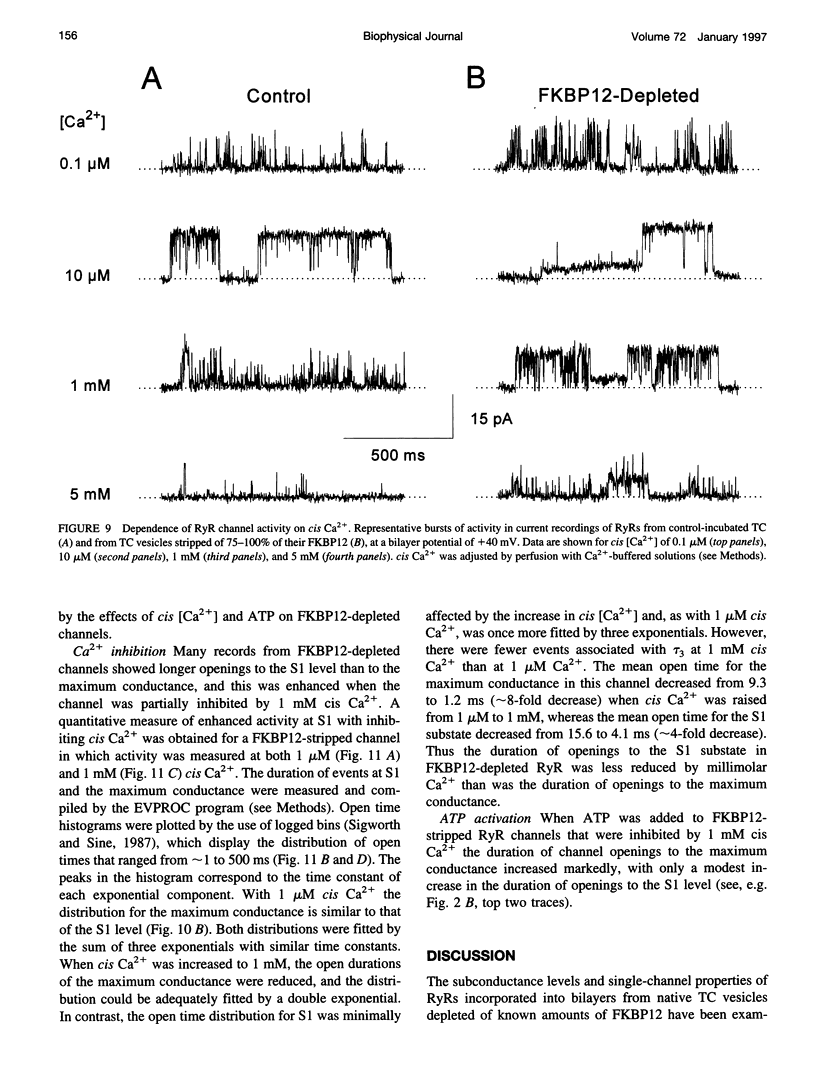
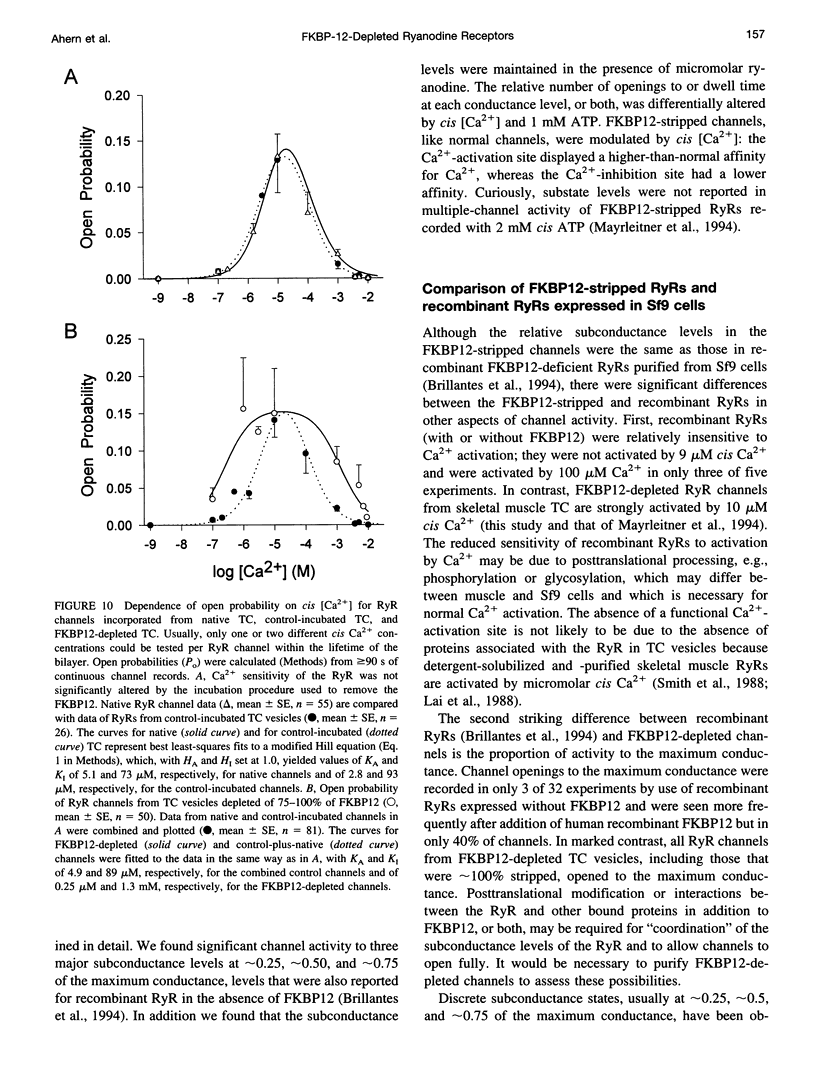
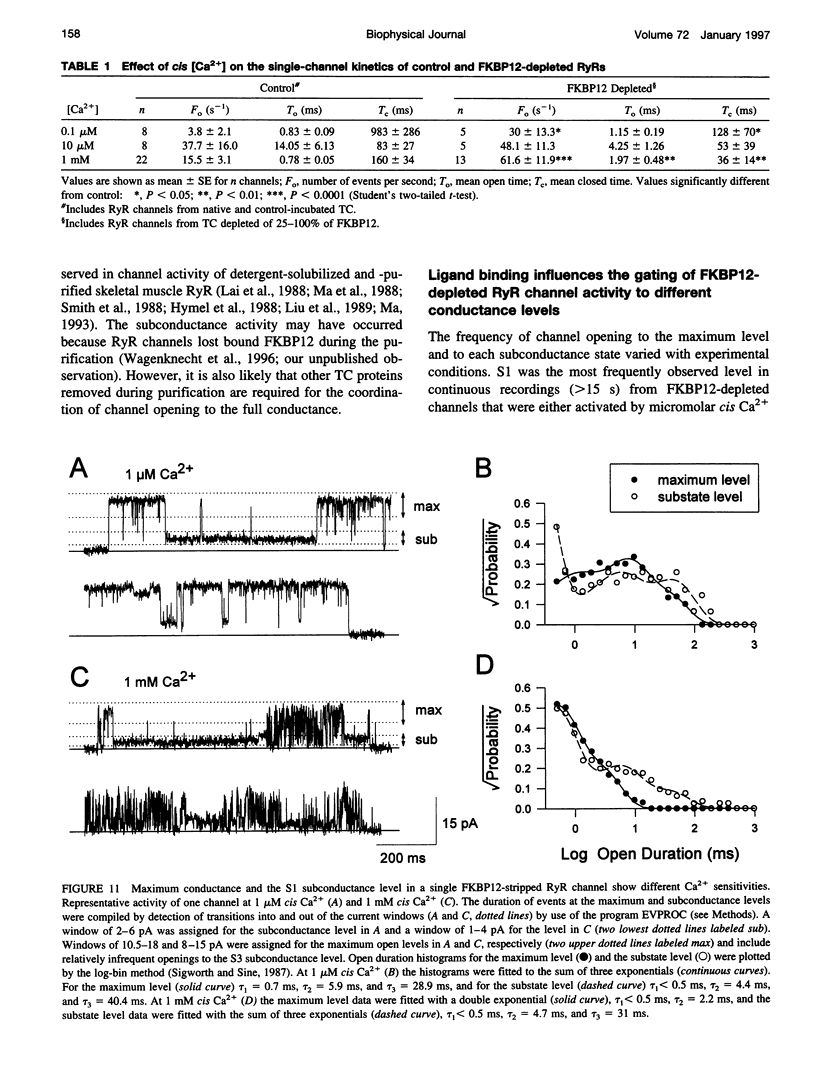
FKBP12 was removed from ryanodine receptors (RyRs) by incubation of rabbit skeletal muscle terminal cisternae membranes with rapamycin. The extent of FKBP12 removal was estimated by immunostaining Western blots of terminal cisternae proteins. Single FKBP12-depleted RyR channels, incorporated into planar lipid bilayers, were modulated by Ca2+, ATP, ryanodine, and ruthenium red in the cis chamber and opened frequently to the normal maximum conductance of approximately 230 pS and to substate levels of approximately 0.25, approximately 0.5, and approximately 0.75 of the maximum conductance. Substate activity was rarely seen in native RyRs. Ryanodine did not after the number of conductance levels in FKBP12-depleted channels, but, at a membrane potential of +40 mV, reduced both the maximum and the substate conductances by approximately 50%. FKBP12-stripped channels were activated by a 10-fold-lower [Ca2+] and inhibited by a 10-fold-higher [Ca2+], than RyRs from control-incubated and native terminal cisternae vesicles. The open probability (Po) of these FKBP12-deficient channels was greater than that of control channels at 0.1 microM and 1 mM cis Ca2+ but no different at 10 microM cis Ca2+, where channels showed maximal Ca2+ activation. The approximately 0.25 substate was less sensitive than the maximum conductance to inhibition by Ca2+ and was the dominant level in channels inhibited by 1 mM cis Ca2+. The results show that FKBP12 coordinates the gating of channel activity in control and ryanodine-modified RyRs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern G. P., Junankar P. R., Dulhunty A. F. Single channel activity of the ryanodine receptor calcium release channel is modulated by FK-506. FEBS Lett. 1994 Oct 3;352(3):369–374. doi: 10.1016/0014-5793(94)01001-3. [DOI] [PubMed] [Google Scholar]
- Bang H., Müller W., Hans M., Brune K., Swandulla D. Activation of Ca2+ signaling in neutrophils by the mast cell-released immunophilin FKBP12. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3435–3438. doi: 10.1073/pnas.92.8.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block B. A., Imagawa T., Campbell K. P., Franzini-Armstrong C. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol. 1988 Dec;107(6 Pt 2):2587–2600. doi: 10.1083/jcb.107.6.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillantes A. B., Ondrias K., Scott A., Kobrinsky E., Ondriasová E., Moschella M. C., Jayaraman T., Landers M., Ehrlich B. E., Marks A. R. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994 May 20;77(4):513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Buck E., Zimanyi I., Abramson J. J., Pessah I. N. Ryanodine stabilizes multiple conformational states of the skeletal muscle calcium release channel. J Biol Chem. 1992 Nov 25;267(33):23560–23567. [PubMed] [Google Scholar]
- Cameron A. M., Steiner J. P., Sabatini D. M., Kaplin A. I., Walensky L. D., Snyder S. H. Immunophilin FK506 binding protein associated with inositol 1,4,5-trisphosphate receptor modulates calcium flux. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1784–1788. doi: 10.1073/pnas.92.5.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell A. H., Brandt N. R., Brunschwig J. P., Purkerson S. Localization and partial characterization of the oligomeric disulfide-linked molecular weight 95,000 protein (triadin) which binds the ryanodine and dihydropyridine receptors in skeletal muscle triadic vesicles. Biochemistry. 1991 Jul 30;30(30):7507–7513. doi: 10.1021/bi00244a020. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. Effects of glycerol treatment and maintained depolarization on charge movement in skeletal muscle. J Physiol. 1976 Jan;254(2):285–316. doi: 10.1113/jphysiol.1976.sp011233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A., Díaz-Muñoz M., Hawkes M. J., Brush K., Hamilton S. L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol Pharmacol. 1990 May;37(5):735–741. [PubMed] [Google Scholar]
- Collins J. H., Tarcsafalvi A., Ikemoto N. Identification of a region of calsequestrin that binds to the junctional face membrane of sarcoplasmic reticulum. Biochem Biophys Res Commun. 1990 Feb 28;167(1):189–193. doi: 10.1016/0006-291x(90)91749-i. [DOI] [PubMed] [Google Scholar]
- Dulhunty A. F. The voltage-activation of contraction in skeletal muscle. Prog Biophys Mol Biol. 1992;57(3):181–223. doi: 10.1016/0079-6107(92)90024-z. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993 Sep 15;216(3):689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Guo W., Campbell K. P. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem. 1995 Apr 21;270(16):9027–9030. doi: 10.1074/jbc.270.16.9027. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto N., Ronjat M., Mészáros L. G., Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989 Aug 8;28(16):6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Jayaraman T., Brillantes A. M., Timerman A. P., Fleischer S., Erdjument-Bromage H., Tempst P., Marks A. R. FK506 binding protein associated with the calcium release channel (ryanodine receptor). J Biol Chem. 1992 May 15;267(14):9474–9477. [PubMed] [Google Scholar]
- Kaftan E., Marks A. R., Ehrlich B. E. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circ Res. 1996 Jun;78(6):990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- Kay J. E. Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochem J. 1996 Mar 1;314(Pt 2):361–385. [PMC free article] [PubMed] [Google Scholar]
- Kourie J. I., Laver D. R., Ahern G. P., Dulhunty A. F. A calcium-activated chloride channel in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Am J Physiol. 1996 Jun;270(6 Pt 1):C1675–C1686. doi: 10.1152/ajpcell.1996.270.6.C1675. [DOI] [PubMed] [Google Scholar]
- Kourie J. I., Laver D. R., Junankar P. R., Gage P. W., Dulhunty A. F. Characteristics of two types of chloride channel in sarcoplasmic reticulum vesicles from rabbit skeletal muscle. Biophys J. 1996 Jan;70(1):202–221. doi: 10.1016/S0006-3495(96)79564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Stephenson D. G. Effects of FK506 and rapamycin on excitation-contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1996 Jul 15;494(Pt 2):569–576. doi: 10.1113/jphysiol.1996.sp021514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver D. R., Roden L. D., Ahern G. P., Eager K. R., Junankar P. R., Dulhunty A. F. Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol. 1995 Sep;147(1):7–22. doi: 10.1007/BF00235394. [DOI] [PubMed] [Google Scholar]
- Leiva M. C., Lyttle C. R. Leukocyte chemotactic activity of FKBP and inhibition by FK506. Biochem Biophys Res Commun. 1992 Jul 31;186(2):1178–1183. doi: 10.1016/0006-291x(92)90871-h. [DOI] [PubMed] [Google Scholar]
- Liu Q. Y., Lai F. A., Rousseau E., Jones R. V., Meissner G. Multiple conductance states of the purified calcium release channel complex from skeletal sarcoplasmic reticulum. Biophys J. 1989 Mar;55(3):415–424. doi: 10.1016/S0006-3495(89)82835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Bhat M. B., Zhao J. Rectification of skeletal muscle ryanodine receptor mediated by FK506 binding protein. Biophys J. 1995 Dec;69(6):2398–2404. doi: 10.1016/S0006-3495(95)80109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. Block by ruthenium red of the ryanodine-activated calcium release channel of skeletal muscle. J Gen Physiol. 1993 Dec;102(6):1031–1056. doi: 10.1085/jgp.102.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. Desensitization of the skeletal muscle ryanodine receptor: evidence for heterogeneity of calcium release channels. Biophys J. 1995 Mar;68(3):893–899. doi: 10.1016/S0006-3495(95)80265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Fill M., Knudson C. M., Campbell K. P., Coronado R. Ryanodine receptor of skeletal muscle is a gap junction-type channel. Science. 1988 Oct 7;242(4875):99–102. doi: 10.1126/science.2459777. [DOI] [PubMed] [Google Scholar]
- Ma J., Zhao J. Highly cooperative and hysteretic response of the skeletal muscle ryanodine receptor to changes in proton concentrations. Biophys J. 1994 Aug;67(2):626–633. doi: 10.1016/S0006-3495(94)80522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrleitner M., Timerman A. P., Wiederrecht G., Fleischer S. The calcium release channel of sarcoplasmic reticulum is modulated by FK-506 binding protein: effect of FKBP-12 on single channel activity of the skeletal muscle ryanodine receptor. Cell Calcium. 1994 Feb;15(2):99–108. doi: 10.1016/0143-4160(94)90048-5. [DOI] [PubMed] [Google Scholar]
- McGrew S. G., Wolleben C., Siegl P., Inui M., Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry. 1989 Feb 21;28(4):1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., Lüttgau H. C. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim Biophys Acta. 1995 May 8;1241(1):59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Menegazzi P., Larini F., Treves S., Guerrini R., Quadroni M., Zorzato F. Identification and characterization of three calmodulin binding sites of the skeletal muscle ryanodine receptor. Biochemistry. 1994 Aug 9;33(31):9078–9084. doi: 10.1021/bi00197a008. [DOI] [PubMed] [Google Scholar]
- Orlova E. V., Serysheva I. I., van Heel M., Hamilton S. L., Chiu W. Two structural configurations of the skeletal muscle calcium release channel. Nat Struct Biol. 1996 Jun;3(6):547–552. doi: 10.1038/nsb0696-547. [DOI] [PubMed] [Google Scholar]
- Patlak J. B. Sodium channel subconductance levels measured with a new variance-mean analysis. J Gen Physiol. 1988 Oct;92(4):413–430. doi: 10.1085/jgp.92.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher M., Rao V., Grassucci R., Frank J., Timerman A. P., Fleischer S., Wagenknecht T. Cryo-electron microscopy and three-dimensional reconstruction of the calcium release channel/ryanodine receptor from skeletal muscle. J Cell Biol. 1994 Oct;127(2):411–423. doi: 10.1083/jcb.127.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermacher M., Wagenknecht T., Grassucci R., Frank J., Inui M., Chadwick C., Fleischer S. Cryo-EM of the native structure of the calcium release channel/ryanodine receptor from sarcoplasmic reticulum. Biophys J. 1992 Apr;61(4):936–940. doi: 10.1016/S0006-3495(92)81900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik L. M., Maines S. L., Ryan D. E., Levin W., Bandiera S., Thomas P. E. A simple, non-chromatographic purification procedure for monoclonal antibodies. Isolation of monoclonal antibodies against cytochrome P450 isozymes. J Immunol Methods. 1987 Jun 26;100(1-2):123–130. doi: 10.1016/0022-1759(87)90180-3. [DOI] [PubMed] [Google Scholar]
- Ríos E., Pizarro G., Stefani E. Charge movement and the nature of signal transduction in skeletal muscle excitation-contraction coupling. Annu Rev Physiol. 1992;54:109–133. doi: 10.1146/annurev.ph.54.030192.000545. [DOI] [PubMed] [Google Scholar]
- Saito A., Seiler S., Chu A., Fleischer S. Preparation and morphology of sarcoplasmic reticulum terminal cisternae from rabbit skeletal muscle. J Cell Biol. 1984 Sep;99(3):875–885. doi: 10.1083/jcb.99.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Serysheva I. I., Orlova E. V., Chiu W., Sherman M. B., Hamilton S. L., van Heel M. Electron cryomicroscopy and angular reconstitution used to visualize the skeletal muscle calcium release channel. Nat Struct Biol. 1995 Jan;2(1):18–24. doi: 10.1038/nsb0195-18. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A. J. The gating of the sheep skeletal sarcoplasmic reticulum Ca(2+)-release channel is regulated by luminal Ca2+. J Membr Biol. 1995 Jul;146(2):133–144. doi: 10.1007/BF00238004. [DOI] [PubMed] [Google Scholar]
- Smith J. S., Coronado R., Meissner G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+. J Gen Physiol. 1986 Nov;88(5):573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Takeda K., Trautmann A. A patch-clamp study of the partial agonist actions of tubocurarine on rat myotubes. J Physiol. 1984 Apr;349:353–374. doi: 10.1113/jphysiol.1984.sp015160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timerman A. P., Jayaraman T., Wiederrecht G., Onoue H., Marks A. R., Fleischer S. The ryanodine receptor from canine heart sarcoplasmic reticulum is associated with a novel FK-506 binding protein. Biochem Biophys Res Commun. 1994 Jan 28;198(2):701–706. doi: 10.1006/bbrc.1994.1101. [DOI] [PubMed] [Google Scholar]
- Timerman A. P., Wiederrecht G., Marcy A., Fleischer S. Characterization of an exchange reaction between soluble FKBP-12 and the FKBP.ryanodine receptor complex. Modulation by FKBP mutants deficient in peptidyl-prolyl isomerase activity. J Biol Chem. 1995 Feb 10;270(6):2451–2459. doi: 10.1074/jbc.270.6.2451. [DOI] [PubMed] [Google Scholar]
- Tinker A., Lindsay A. R., Williams A. J. A model for ionic conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J Gen Physiol. 1992 Sep;100(3):495–517. doi: 10.1085/jgp.100.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A., Xu L., Mann G., Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys J. 1995 Jul;69(1):106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyerman S. D., Terry B. R., Findlay G. P. Multiple conductances in the large K+ channel from Chara corallina shown by a transient analysis method. Biophys J. 1992 Mar;61(3):736–749. doi: 10.1016/S0006-3495(92)81878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., Berkowitz J., Grassucci R., Timerman A. P., Fleischer S. Localization of calmodulin binding sites on the ryanodine receptor from skeletal muscle by electron microscopy. Biophys J. 1994 Dec;67(6):2286–2295. doi: 10.1016/S0006-3495(94)80714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Berkowitz J., Wiederrecht G. J., Xin H. B., Fleischer S. Cryoelectron microscopy resolves FK506-binding protein sites on the skeletal muscle ryanodine receptor. Biophys J. 1996 Apr;70(4):1709–1715. doi: 10.1016/S0006-3495(96)79733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T., Radermacher M. Three-dimensional architecture of the skeletal muscle ryanodine receptor. FEBS Lett. 1995 Aug 1;369(1):43–46. doi: 10.1016/0014-5793(95)00581-s. [DOI] [PubMed] [Google Scholar]
- Wang T., Donahoe P. K., Zervos A. S. Specific interaction of type I receptors of the TGF-beta family with the immunophilin FKBP-12. Science. 1994 Jul 29;265(5172):674–676. doi: 10.1126/science.7518616. [DOI] [PubMed] [Google Scholar]