Abstract
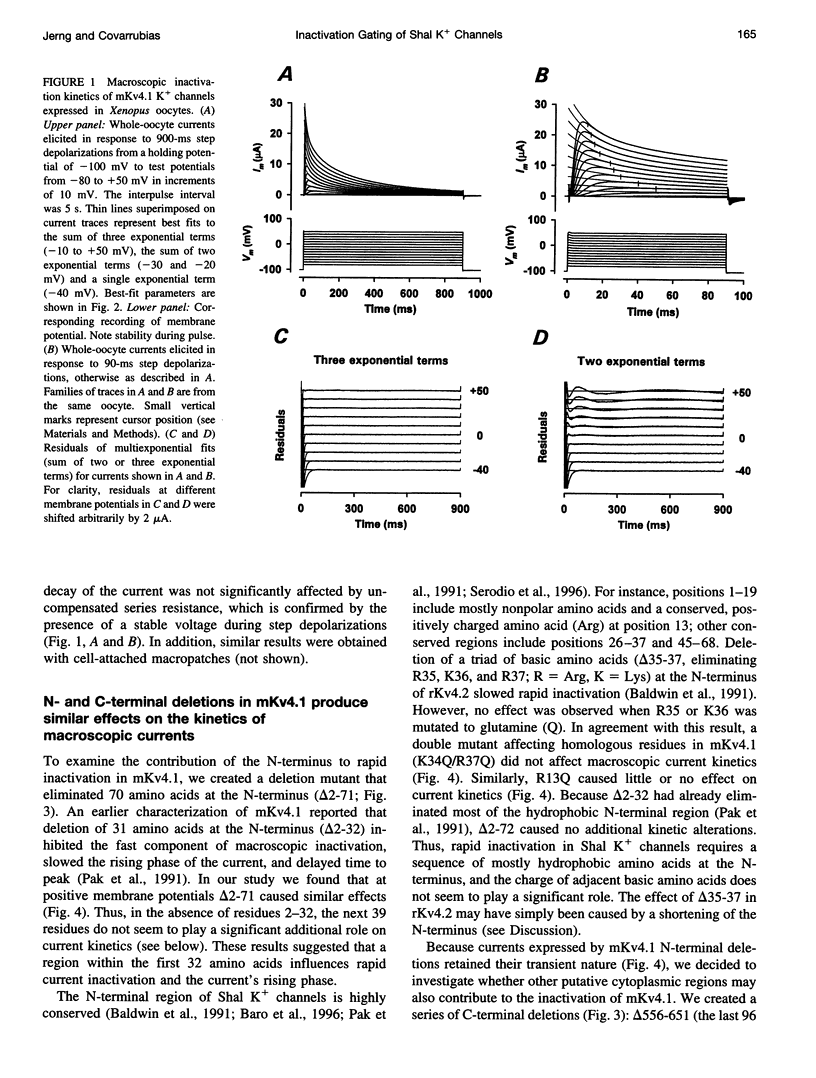
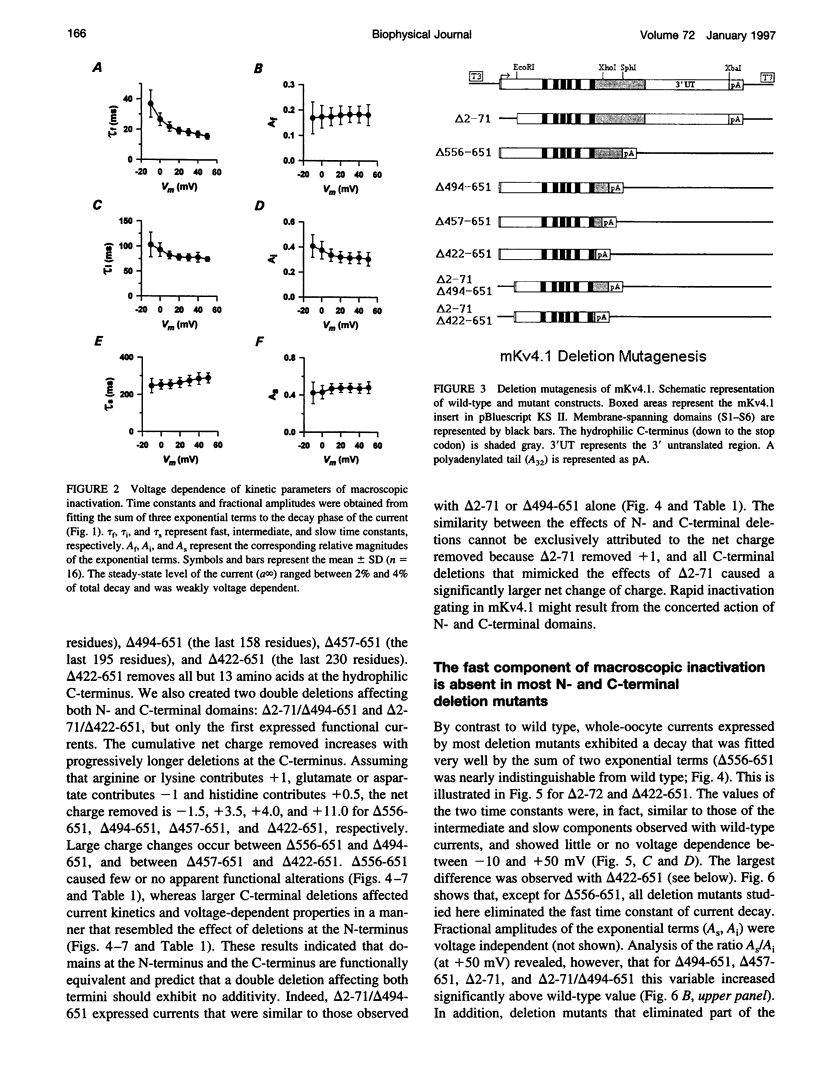
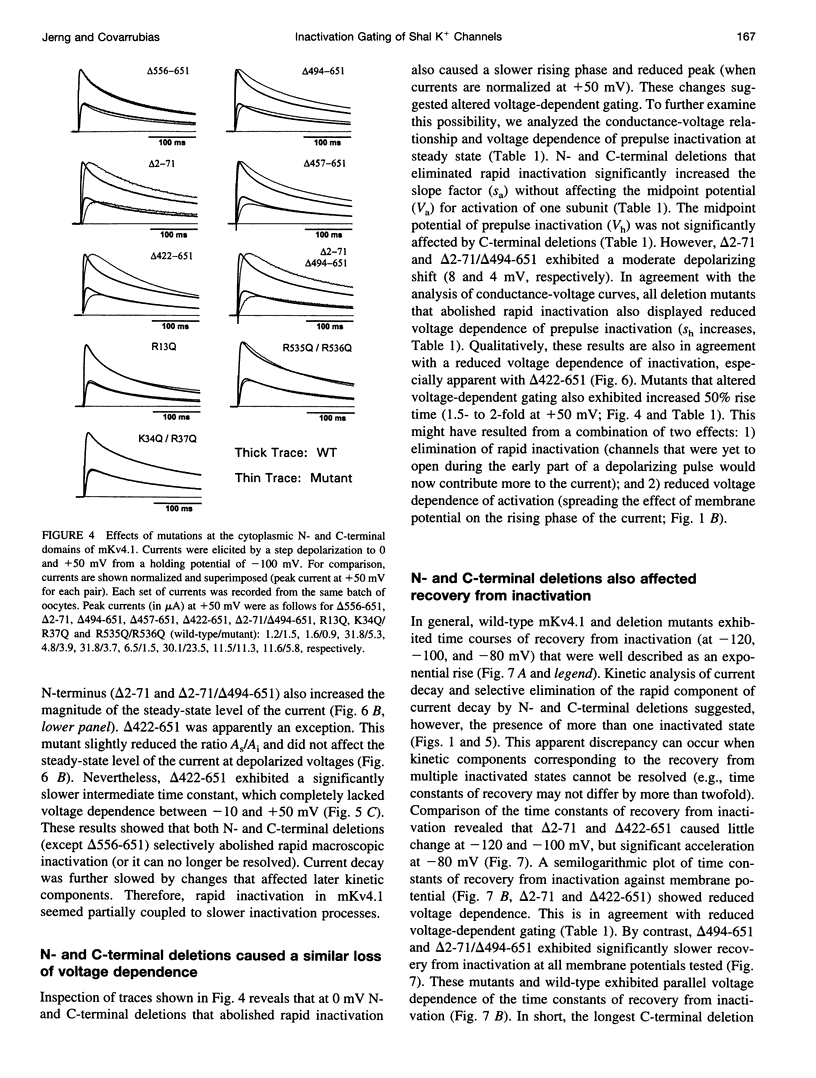
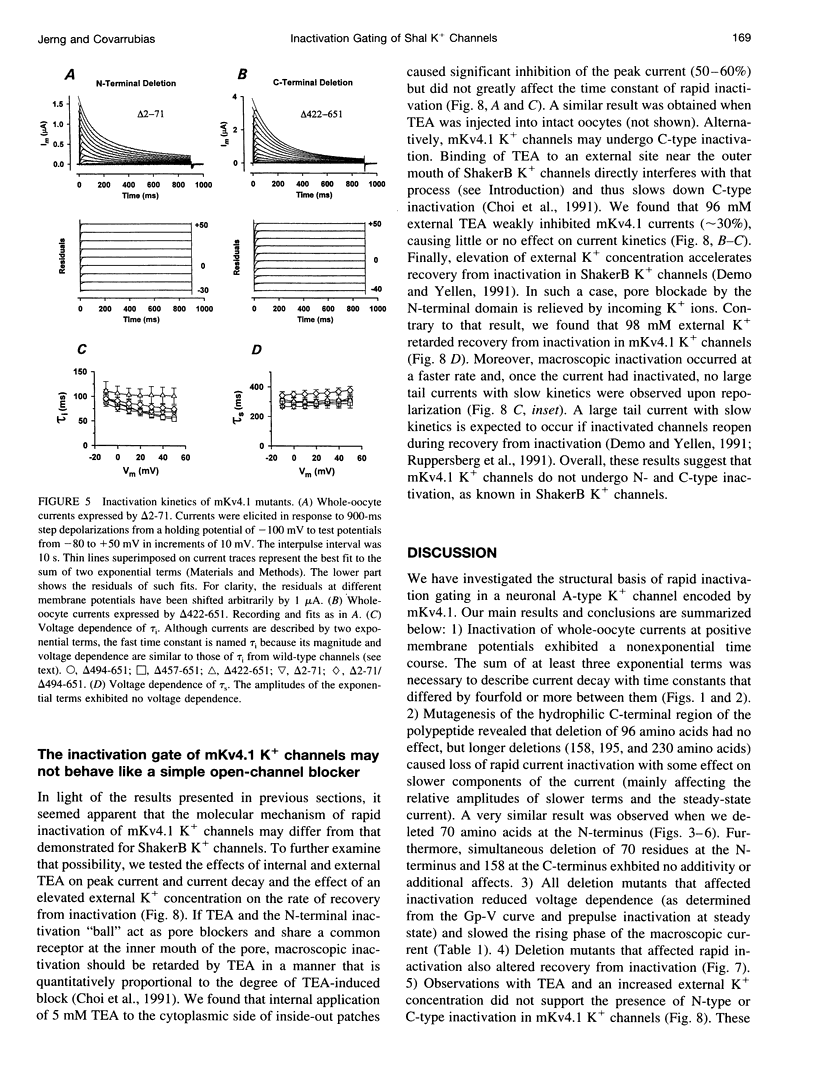
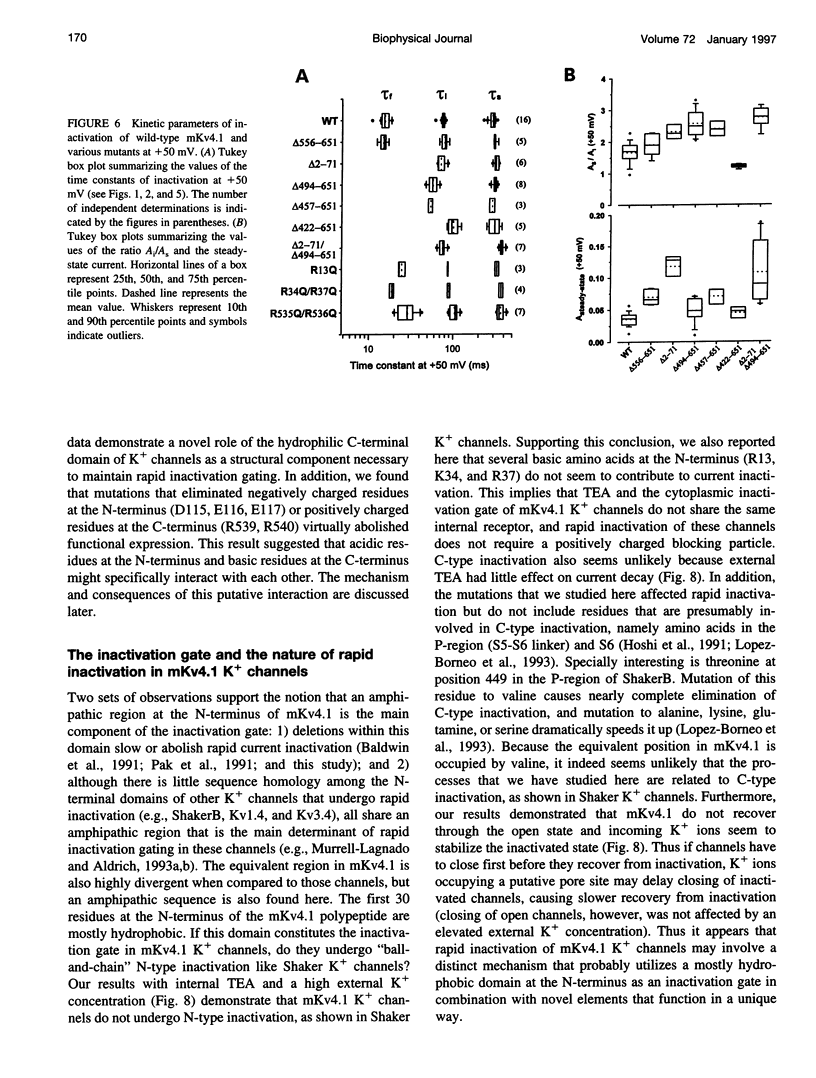
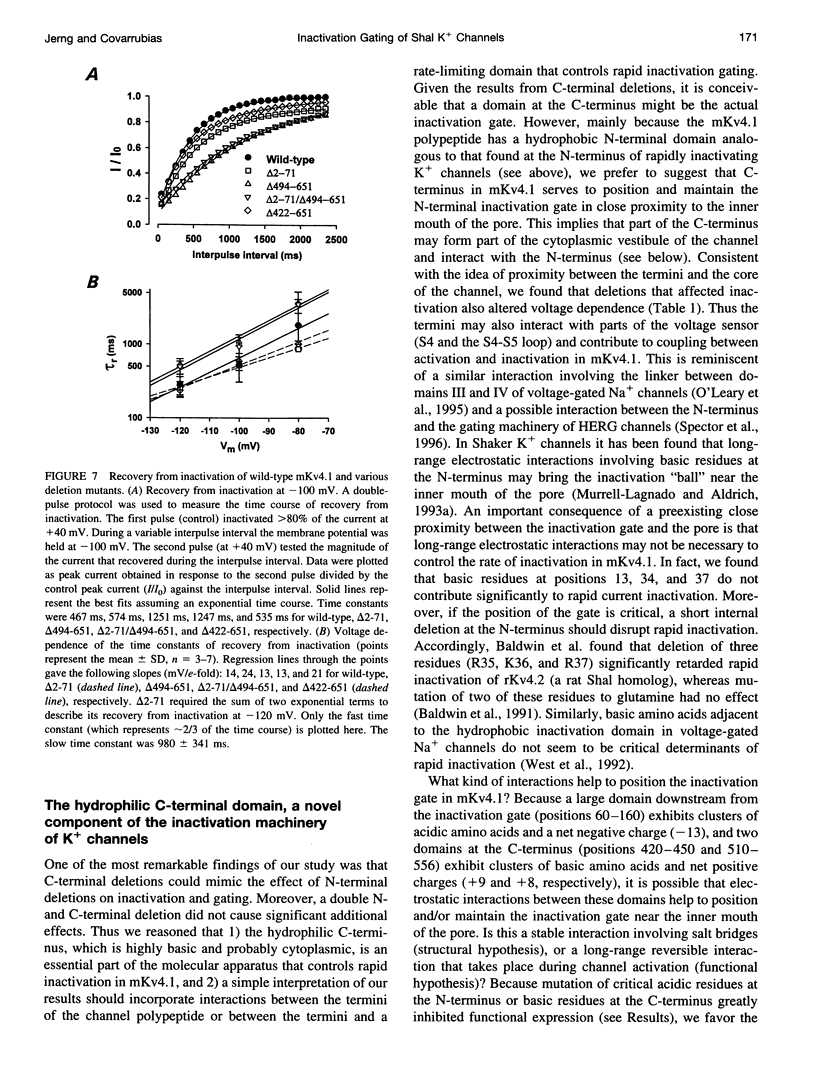
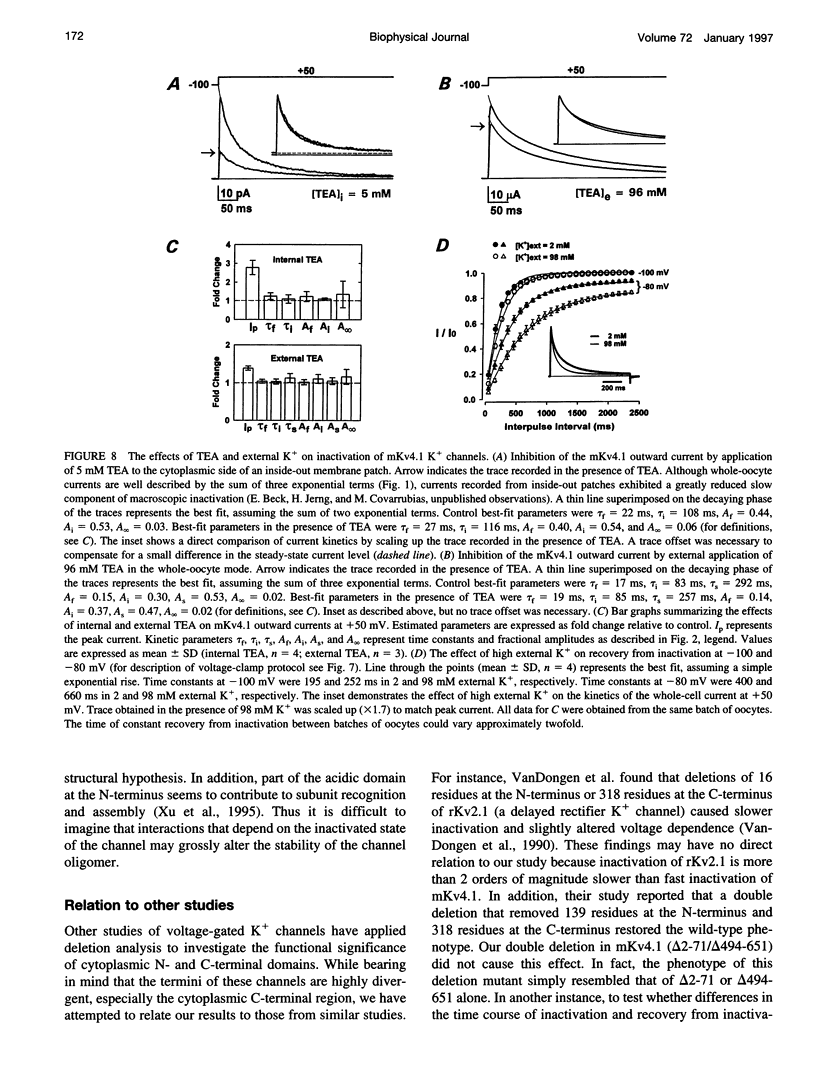
We have examined the molecular mechanism of rapid inactivation gating in a mouse Shal K+ channel (mKv4.1). The results showed that inactivation of these channels follows a complex time course that is well approximated by the sum of three exponential terms. Truncation of an amphipathic region at the N-terminus (residues 2-71) abolished the rapid phase of inactivation (r = 16 ms) and altered voltage-dependent gating. Surprisingly, these effects could be mimicked by deletions affecting the hydrophilic C-terminus. The sum of two exponential terms was sufficient to describe the inactivation of deletion mutants. In fact, the time constants corresponded closely to those of the intermediate and slow phases of inactivation observed with wild-type channels. Further analysis revealed that several basic amino acids at the N-terminus do not influence inactivation, but a positively charged domain at the C-terminus (amino acids 420-550) is necessary to support rapid inactivation. Thus, the amphipathic N-terminus and the hydrophilic C-terminus of mKv4.1 are essential determinants of inactivation gating and may interact with each other to maintain the N-terminal inactivation gate near the inner mouth of the channel. Furthermore, this inactivation gate may not behave like a simple open-channel blocker because channel blockade by internal tetraethylammonium was not associated with slower current decay and an elevated external K+ concentration retarded recovery from inactivation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Tsaur M. L., Lopez G. A., Jan Y. N., Jan L. Y. Characterization of a mammalian cDNA for an inactivating voltage-sensitive K+ channel. Neuron. 1991 Sep;7(3):471–483. doi: 10.1016/0896-6273(91)90299-f. [DOI] [PubMed] [Google Scholar]
- Baro D. J., Coniglio L. M., Cole C. L., Rodriguez H. E., Lubell J. K., Kim M. T., Harris-Warrick R. M. Lobster shal: comparison with Drosophila shal and native potassium currents in identified neurons. J Neurosci. 1996 Mar 1;16(5):1689–1701. doi: 10.1523/JNEUROSCI.16-05-01689.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995 Oct;15(4):951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Chabala L. D., Bakry N., Covarrubias M. Low molecular weight poly(A)+ mRNA species encode factors that modulate gating of a non-Shaker A-type K+ channel. J Gen Physiol. 1993 Oct;102(4):713–728. doi: 10.1085/jgp.102.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K. L., Aldrich R. W., Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M., Wei A., Salkoff L., Vyas T. B. Elimination of rapid potassium channel inactivation by phosphorylation of the inactivation gate. Neuron. 1994 Dec;13(6):1403–1412. doi: 10.1016/0896-6273(94)90425-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron. 1991 Nov;7(5):743–753. doi: 10.1016/0896-6273(91)90277-7. [DOI] [PubMed] [Google Scholar]
- Hopkins W. F., Demas V., Tempel B. L. Both N- and C-terminal regions contribute to the assembly and functional expression of homo- and heteromultimeric voltage-gated K+ channels. J Neurosci. 1994 Mar;14(3 Pt 1):1385–1393. doi: 10.1523/JNEUROSCI.14-03-01385.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 1990 Oct 26;250(4980):533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T., Zagotta W. N., Aldrich R. W. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991 Oct;7(4):547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Iverson L. E., Rudy B. The role of the divergent amino and carboxyl domains on the inactivation properties of potassium channels derived from the Shaker gene of Drosophila. J Neurosci. 1990 Sep;10(9):2903–2916. doi: 10.1523/JNEUROSCI.10-09-02903.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley G. C., Ackerman S. J., John J. E., 3rd, Jones L. R., Moorman J. R. Hyperpolarization-activated chloride currents in Xenopus oocytes. J Gen Physiol. 1994 Feb;103(2):217–230. doi: 10.1085/jgp.103.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jurman M. E., Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996 Apr;16(4):859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- López-Barneo J., Hoshi T., Heinemann S. H., Aldrich R. W. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1(1):61–71. [PubMed] [Google Scholar]
- MacKinnon R., Aldrich R. W., Lee A. W. Functional stoichiometry of Shaker potassium channel inactivation. Science. 1993 Oct 29;262(5134):757–759. doi: 10.1126/science.7694359. [DOI] [PubMed] [Google Scholar]
- Murrell-Lagnado R. D., Aldrich R. W. Energetics of Shaker K channels block by inactivation peptides. J Gen Physiol. 1993 Dec;102(6):977–1003. doi: 10.1085/jgp.102.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell-Lagnado R. D., Aldrich R. W. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J Gen Physiol. 1993 Dec;102(6):949–975. doi: 10.1085/jgp.102.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary M. E., Chen L. Q., Kallen R. G., Horn R. A molecular link between activation and inactivation of sodium channels. J Gen Physiol. 1995 Oct;106(4):641–658. doi: 10.1085/jgp.106.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogielska E. M., Zagotta W. N., Hoshi T., Heinemann S. H., Haab J., Aldrich R. W. Cooperative subunit interactions in C-type inactivation of K channels. Biophys J. 1995 Dec;69(6):2449–2457. doi: 10.1016/S0006-3495(95)80114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak M. D., Baker K., Covarrubias M., Butler A., Ratcliffe A., Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyi G., Sheng Z., Deutsch C. C-type inactivation of a voltage-gated K+ channel occurs by a cooperative mechanism. Biophys J. 1995 Sep;69(3):896–903. doi: 10.1016/S0006-3495(95)79963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppersberg J. P., Frank R., Pongs O., Stocker M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature. 1991 Oct 17;353(6345):657–660. doi: 10.1038/353657a0. [DOI] [PubMed] [Google Scholar]
- Salkoff L., Baker K., Butler A., Covarrubias M., Pak M. D., Wei A. An essential 'set' of K+ channels conserved in flies, mice and humans. Trends Neurosci. 1992 May;15(5):161–166. doi: 10.1016/0166-2236(92)90165-5. [DOI] [PubMed] [Google Scholar]
- Serôdio P., Kentros C., Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol. 1994 Oct;72(4):1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serôdio P., Vega-Saenz de Miera E., Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 1996 May;75(5):2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Shen N. V., Chen X., Boyer M. M., Pfaffinger P. J. Deletion analysis of K+ channel assembly. Neuron. 1993 Jul;11(1):67–76. doi: 10.1016/0896-6273(93)90271-r. [DOI] [PubMed] [Google Scholar]
- Sheng M., Tsaur M. L., Jan Y. N., Jan L. Y. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992 Aug;9(2):271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Spector P. S., Curran M. E., Zou A., Keating M. T., Sanguinetti M. C. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996 May;107(5):611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J., Yao J. A., Berman M. F., Tseng G. N. Functional role of the NH2-terminal cytoplasmic domain of a mammalian A-type K channel. J Gen Physiol. 1993 Dec;102(6):1057–1083. doi: 10.1085/jgp.102.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDongen A. M., Frech G. C., Drewe J. A., Joho R. H., Brown A. M. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990 Oct;5(4):433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yu W., Jan Y. N., Jan L. Y., Li M. Assembly of voltage-gated potassium channels. Conserved hydrophilic motifs determine subfamily-specific interactions between the alpha-subunits. J Biol Chem. 1995 Oct 20;270(42):24761–24768. doi: 10.1074/jbc.270.42.24761. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Hoshi T., Aldrich R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science. 1990 Oct 26;250(4980):568–571. doi: 10.1126/science.2122520. [DOI] [PubMed] [Google Scholar]



