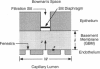
Abstract
The filtrate formed by renal glomerular capillaries must pass through a layer of endothelial cells, the glomerular basement membrane (GBM), and a layer of epithelial cells, arranged in series. To elucidate the relative resistances of the GBM and cell layers to movement of uncharged macromolecules, we measured the diffusional permeabilities of intact and cell-free capillaries to narrow fractions of Ficoll with Stokes-Einstein radii ranging from 3.0 to 6.2 nm. Glomeruli were isolated from rat kidneys, and diffusion of fluorescein-labeled Ficoll across the walls of single capillary loops was monitored with a confocal microscopy technique. In half of the experiments the glomeruli were treated first to remove the cells, leaving skeletons that retained the general shape of the glomerulus and consisted almost entirely of GBM. The diffusional permeability of cell-free capillaries to Ficoll was approximately 10 to 20 times that of intact capillaries, depending on molecular size. Taking into account the blockage of much of the GBM surface by cells, the contribution of the GBM to the diffusional resistance of the intact barrier was calculated to be 13% to 26% of the total, increasing with molecular size. Thus, the GBM contribution, although smaller than that of the cells, was not negligible. The structure that is most likely to be responsible for the cellular part of the diffusional resistance is the slit diaphragm, which spans the filtration slit between epithelial foot processes. A novel hydrodynamic model was developed to relate the diffusional resistance of the slit diaphragm to its structure, which was idealized as a single layer of cylindrical fibers in a ladder-like arrangement.
Full text
PDF
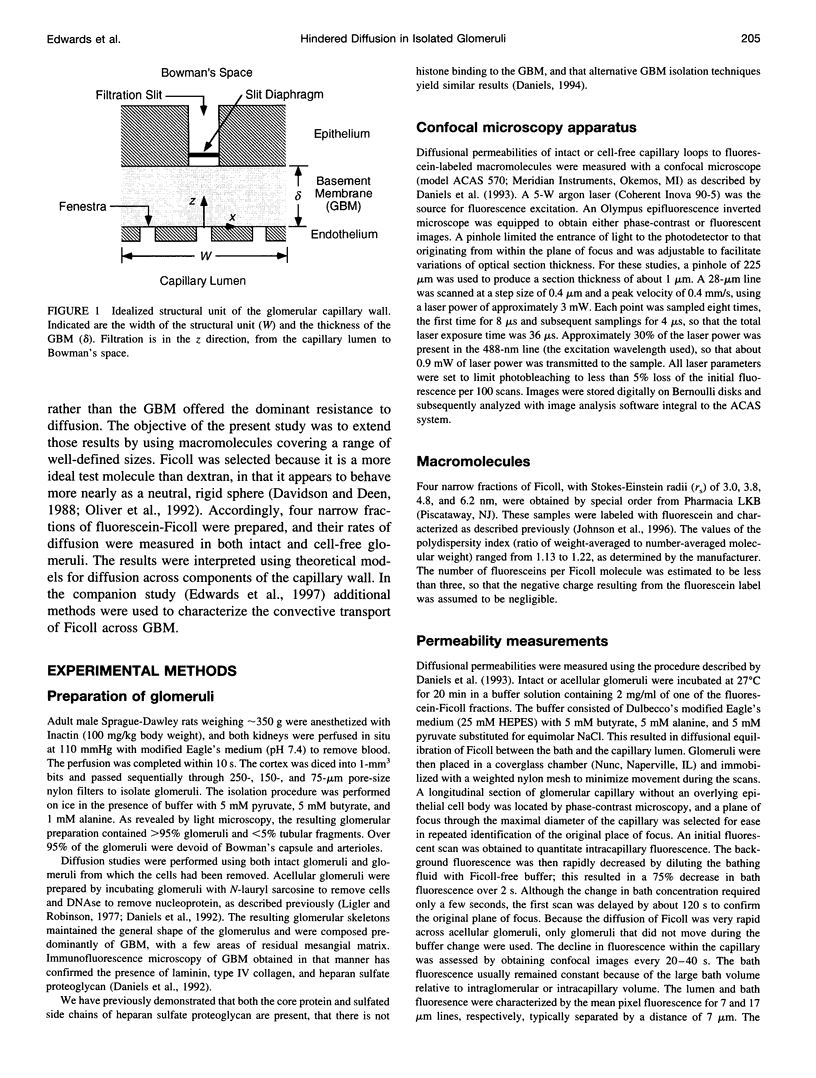
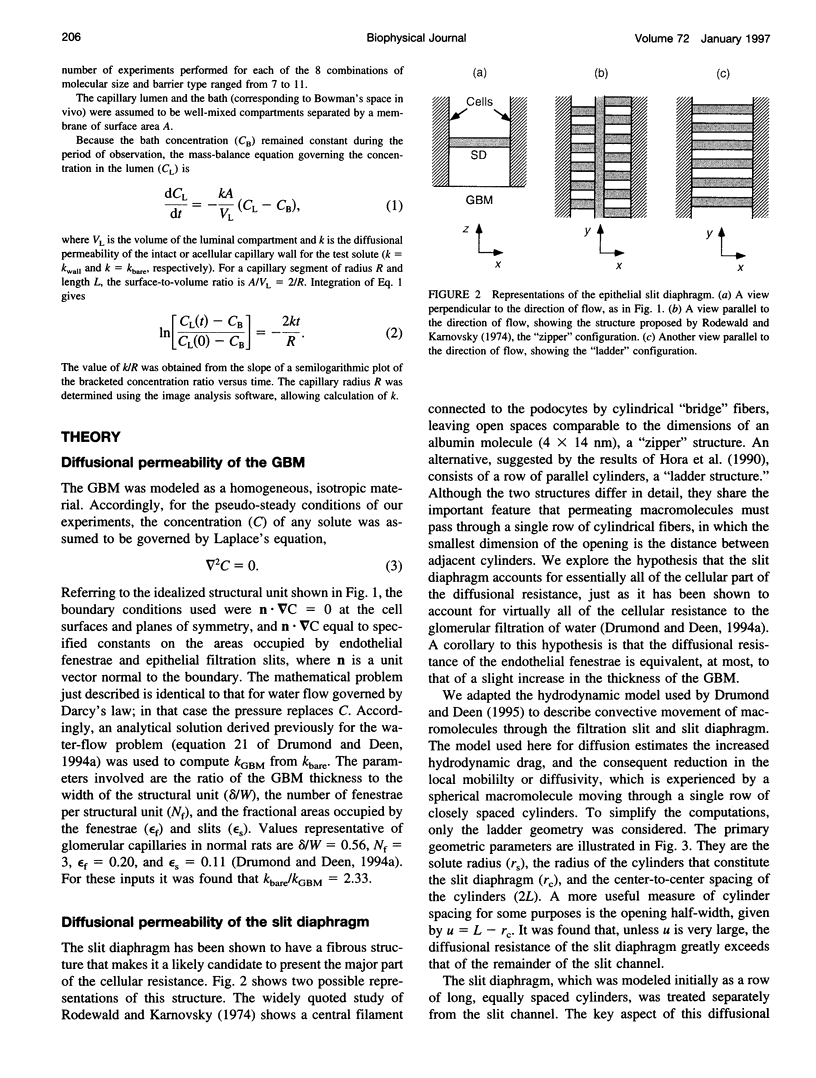
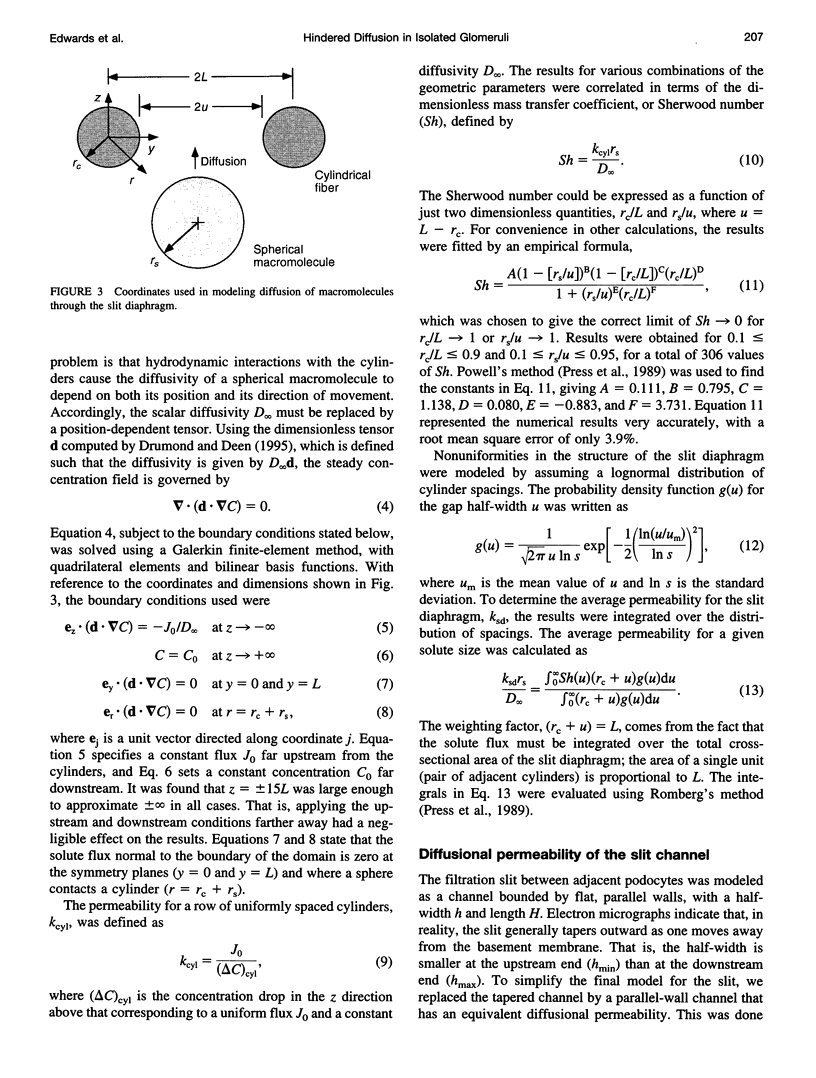
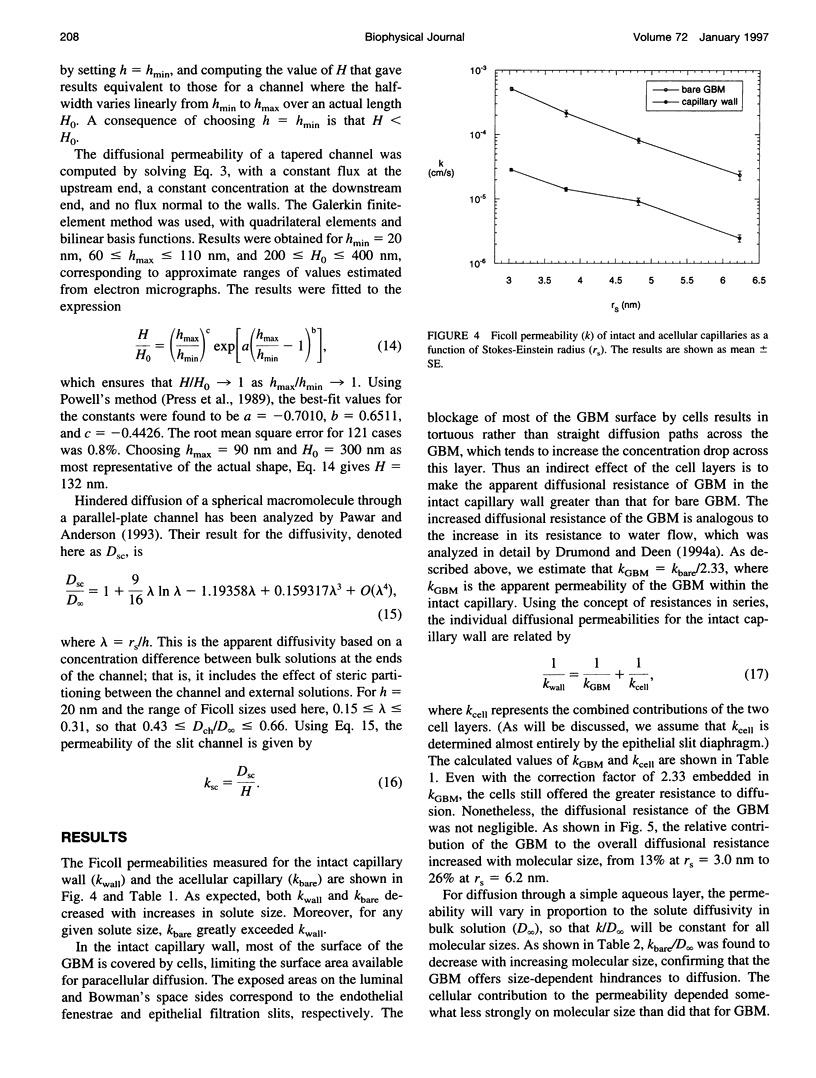
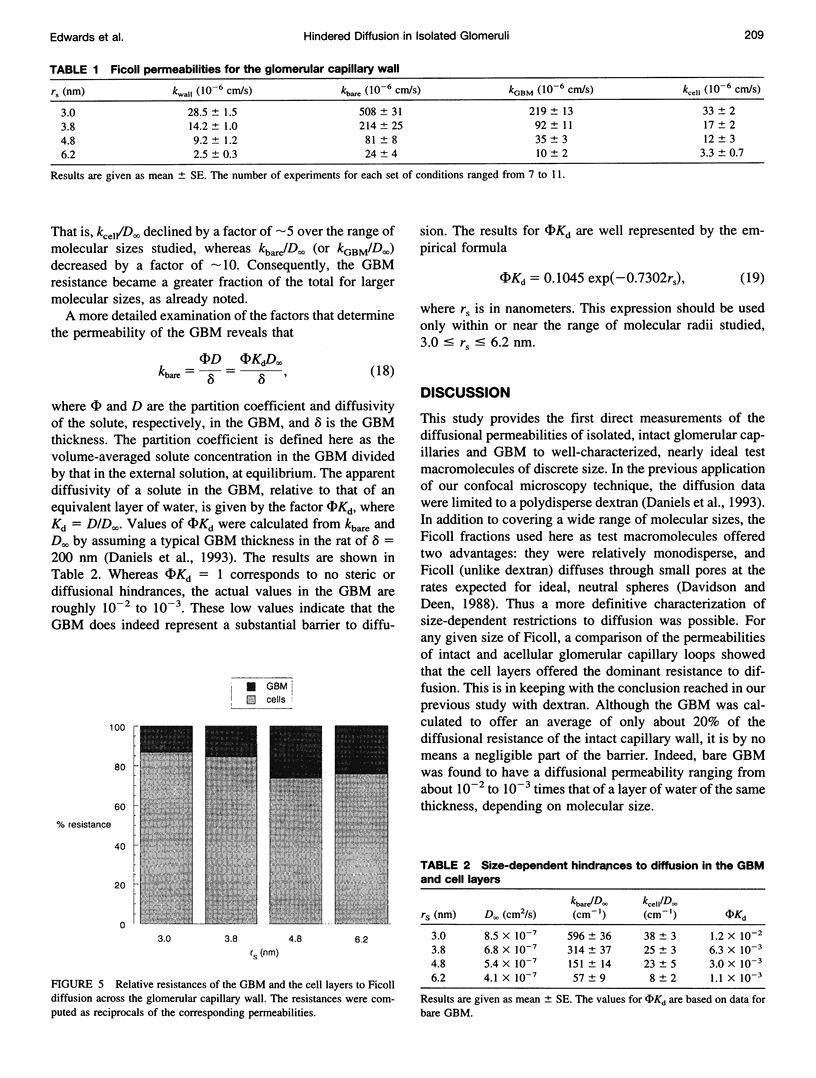
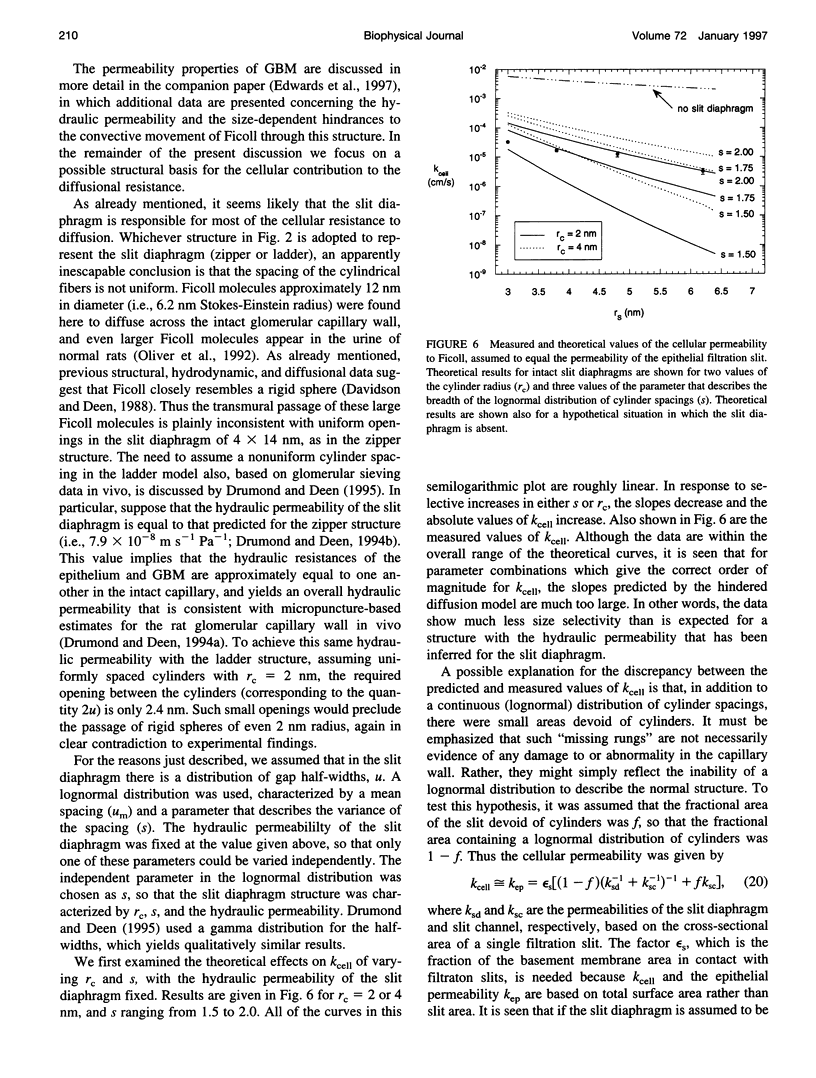
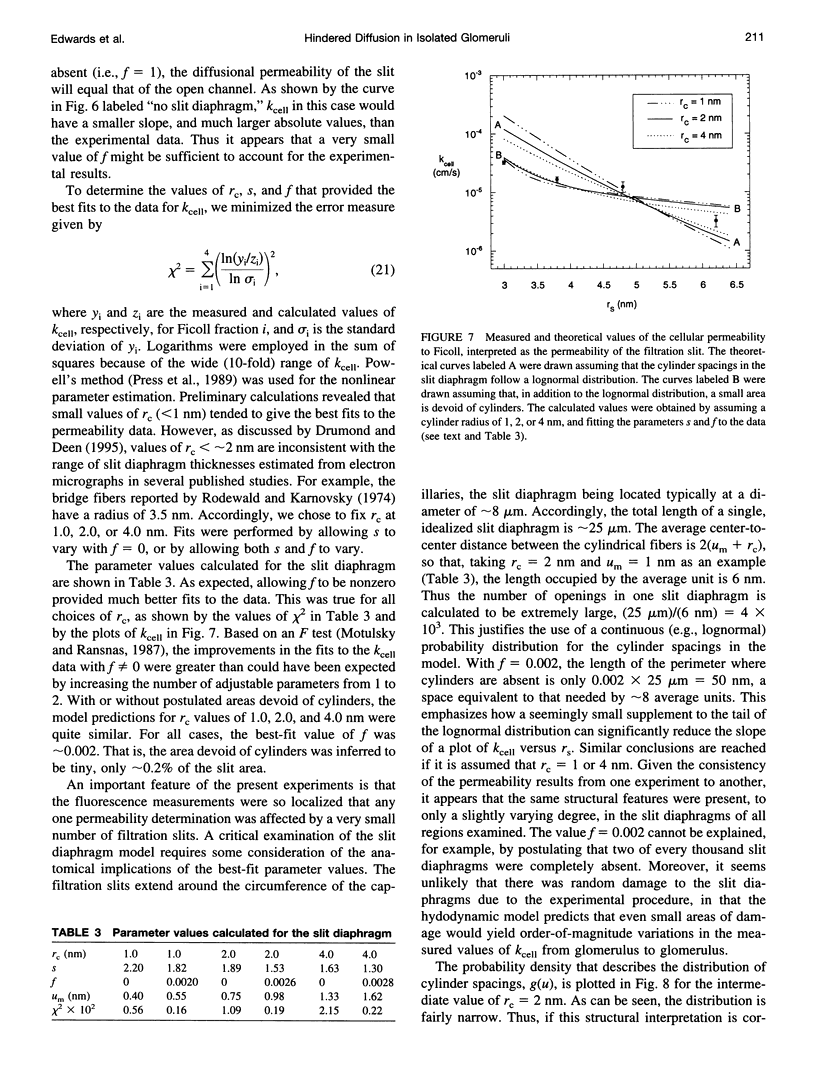

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson R. H., Michel C. C. Pathways through the intercellular clefts of frog mesenteric capillaries. J Physiol. 1993 Jul;466:303–327. [PMC free article] [PubMed] [Google Scholar]
- Bray J., Robinson G. B. Influence of charge on filtration across renal basement membrane films in vitro. Kidney Int. 1984 Mar;25(3):527–533. doi: 10.1038/ki.1984.49. [DOI] [PubMed] [Google Scholar]
- Daniels B. S., Deen W. M., Mayer G., Meyer T., Hostetter T. H. Glomerular permeability barrier in the rat. Functional assessment by in vitro methods. J Clin Invest. 1993 Aug;92(2):929–936. doi: 10.1172/JCI116668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels B. S., Hauser E. B., Deen W. M., Hostetter T. H. Glomerular basement membrane: in vitro studies of water and protein permeability. Am J Physiol. 1992 Jun;262(6 Pt 2):F919–F926. doi: 10.1152/ajprenal.1992.262.6.F919. [DOI] [PubMed] [Google Scholar]
- Daniels B. S. Increased albumin permeability in vitro following alterations of glomerular charge is mediated by the cells of the filtration barrier. J Lab Clin Med. 1994 Aug;124(2):224–230. [PubMed] [Google Scholar]
- Drumond M. C., Deen W. M. Hindered transport of macromolecules through a single row of cylinders: application to glomerular filtration. J Biomech Eng. 1995 Nov;117(4):414–422. doi: 10.1115/1.2794202. [DOI] [PubMed] [Google Scholar]
- Drumond M. C., Deen W. M. Stokes flow through a row of cylinders between parallel walls: model for the glomerular slit diaphragm. J Biomech Eng. 1994 May;116(2):184–189. doi: 10.1115/1.2895718. [DOI] [PubMed] [Google Scholar]
- Drumond M. C., Deen W. M. Structural determinants of glomerular hydraulic permeability. Am J Physiol. 1994 Jan;266(1 Pt 2):F1–12. doi: 10.1152/ajprenal.1994.266.1.F1. [DOI] [PubMed] [Google Scholar]
- Fu B. M., Curry F. E., Weinbaum S. A diffusion wake model for tracer ultrastructure-permeability studies in microvessels. Am J Physiol. 1995 Dec;269(6 Pt 2):H2124–H2140. doi: 10.1152/ajpheart.1995.269.6.H2124. [DOI] [PubMed] [Google Scholar]
- Fu B. M., Weinbaum S., Tsay R. Y., Curry F. E. A junction-orifice-fiber entrance layer model for capillary permeability: application to frog mesenteric capillaries. J Biomech Eng. 1994 Nov;116(4):502–513. doi: 10.1115/1.2895802. [DOI] [PubMed] [Google Scholar]
- Hora K., Ohno S., Oguchi H., Furukawa T., Furuta S. Three-dimensional study of glomerular slit diaphragm by the quick-freezing and deep-etching replica method. Eur J Cell Biol. 1990 Dec;53(2):402–406. [PubMed] [Google Scholar]
- Johnson E. M., Berk D. A., Jain R. K., Deen W. M. Hindered diffusion in agarose gels: test of effective medium model. Biophys J. 1996 Feb;70(2):1017–1023. doi: 10.1016/S0006-3495(96)79645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987 Nov;1(5):365–374. [PubMed] [Google Scholar]
- Oliver J. D., 3rd, Anderson S., Troy J. L., Brenner B. M., Deen W. H. Determination of glomerular size-selectivity in the normal rat with Ficoll. J Am Soc Nephrol. 1992 Aug;3(2):214–228. doi: 10.1681/ASN.V32214. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R., Karnovsky M. J. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974 Feb;60(2):423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]