Abstract
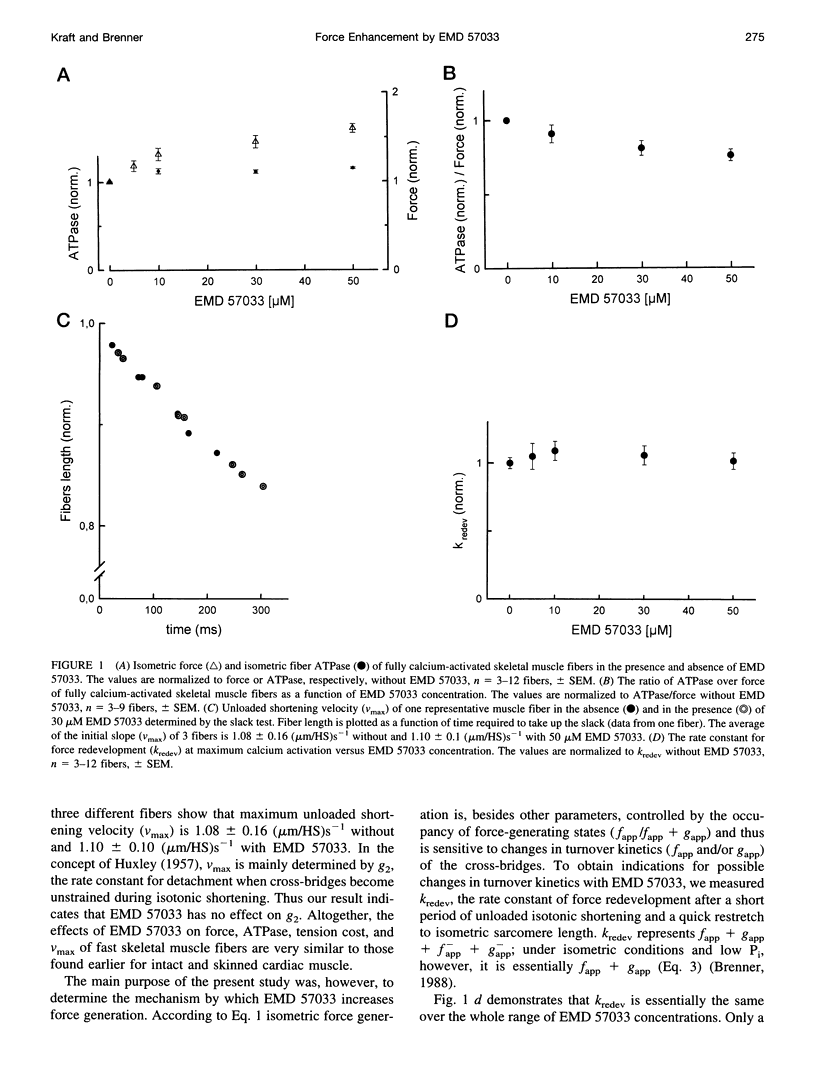
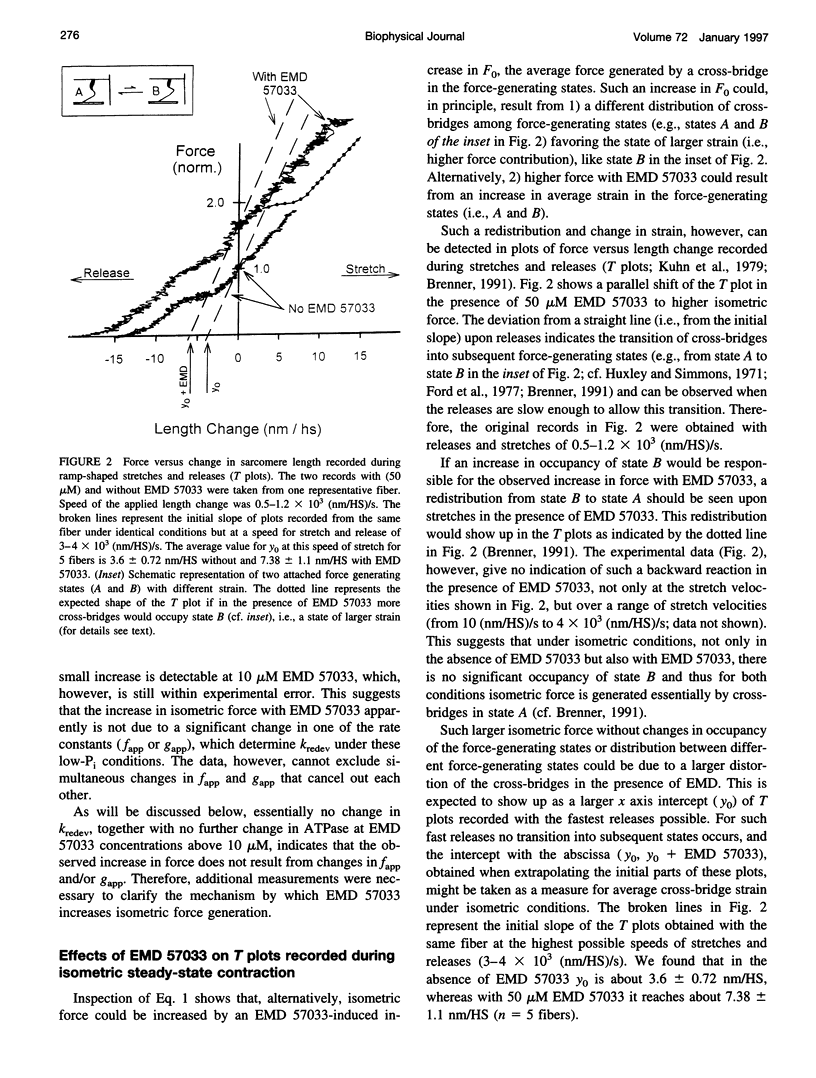
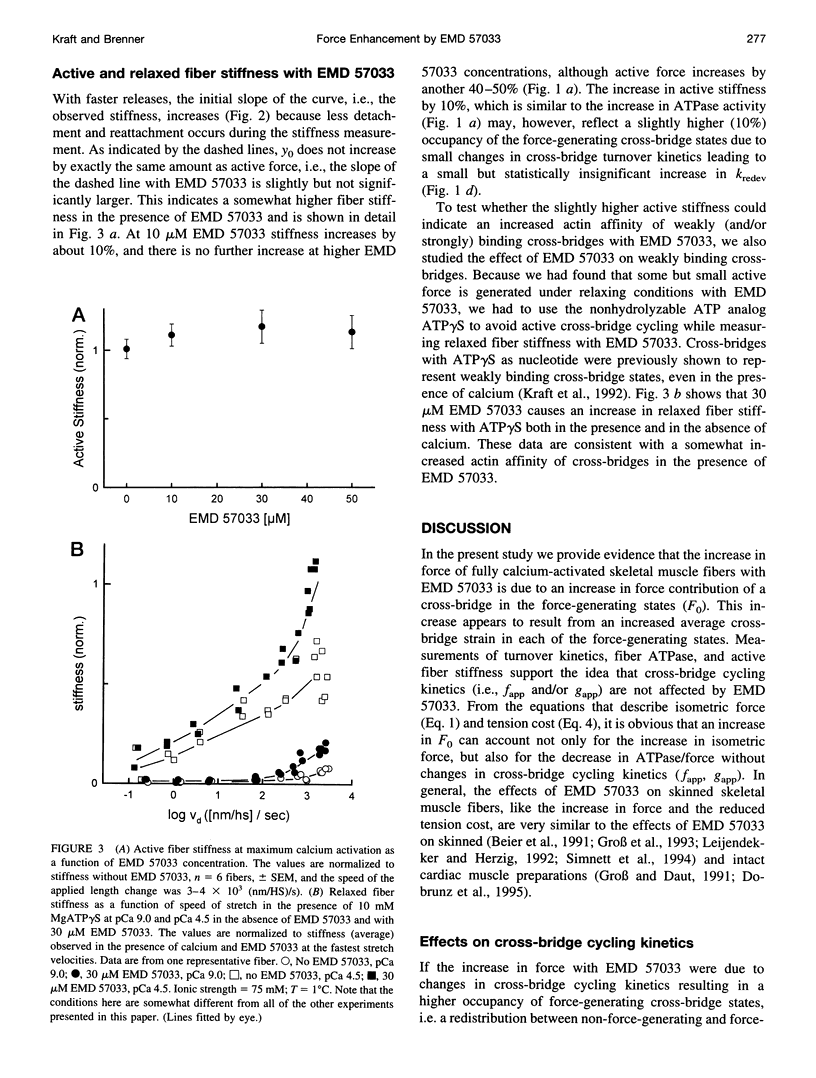
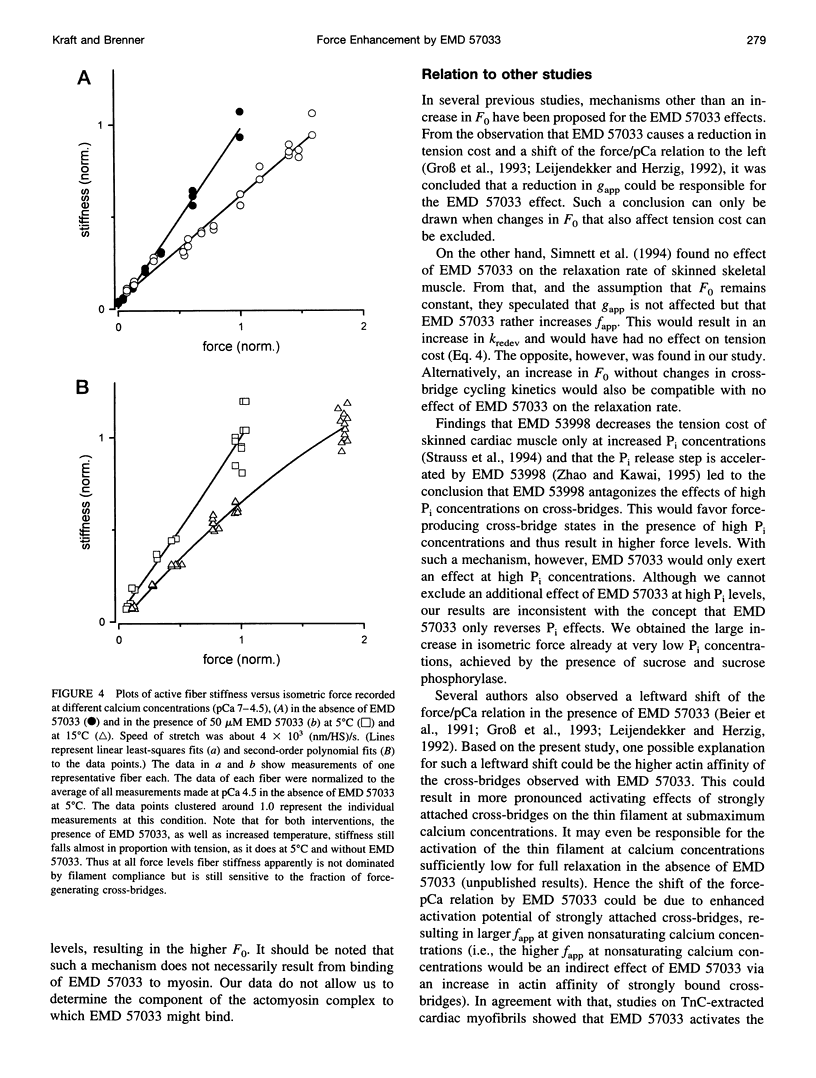
The thiadiazinon derivative EMD 57033 has been found previously in cardiac muscle to increase isometric force generation without a proportional increase in fiber ATPase, thus causing a reduction in tension cost. To analyze the mechanism by which EMD 57033 affects the contractile system, we studied its effects on isometric force, isometric fiber ATPase, the rate constant of force redevelopment (k(redev)), active fiber stiffness, and its effect on Fo, which is the force contribution of a cross-bridge in the force-generating states. We used chemically skinned fibers of the rabbit psoas muscle. It was found that with 50 microM EMD 57033, isometric force increases by more than 50%, whereas Kredev, active stiffness, and isometric fiber ATPase increase by at most 10%. The results show that EMD 57033 causes no changes in cross-bridge turnover kinetics and no changes in active fiber stiffness that would result in a large enough increase in occupancy of the force-generating states to account for the increase in active force. However, plots of force versus length change recorded during stretches and releases (T plots) indicate that in the presence of EMD 57033 the y(o) value (x axis intercept) for the cross-bridges becomes more negative while its absolute value increases. This might suggest a larger cross-bridge strain as the basis for increased active force. Analysis of T plots with and without EMD 57033 shows that the increase in cross-bridge strain is not due to a redistribution of cross-bridges among different force-generating states favoring states of larger strain. Instead, it reflects an increased cross-bridge strain in the main force-generating state. The direct effect of EMD 57033 on the force contribution of cross-bridges in the force-generating states represents an alternative mechanism for a positive inotropic intervention.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beier N., Harting J., Jonas R., Klockow M., Lues I., Haeusler G. The novel cardiotonic agent EMD 53 998 is a potent "calcium sensitizer". J Cardiovasc Pharmacol. 1991 Jul;18(1):17–27. doi: 10.1097/00005344-199107000-00004. [DOI] [PubMed] [Google Scholar]
- Brenner B., Chalovich J. M., Greene L. E., Eisenberg E., Schoenberg M. Stiffness of skinned rabbit psoas fibers in MgATP and MgPPi solution. Biophys J. 1986 Oct;50(4):685–691. doi: 10.1016/S0006-3495(86)83509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Effect of Ca2+ on cross-bridge turnover kinetics in skinned single rabbit psoas fibers: implications for regulation of muscle contraction. Proc Natl Acad Sci U S A. 1988 May;85(9):3265–3269. doi: 10.1073/pnas.85.9.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc Natl Acad Sci U S A. 1986 May;83(10):3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Rapid dissociation and reassociation of actomyosin cross-bridges during force generation: a newly observed facet of cross-bridge action in muscle. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10490–10494. doi: 10.1073/pnas.88.23.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. Technique for stabilizing the striation pattern in maximally calcium-activated skinned rabbit psoas fibers. Biophys J. 1983 Jan;41(1):99–102. doi: 10.1016/S0006-3495(83)84411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz L. E., Backx P. H., Yue D. T. Steady-state [Ca2+]i-force relationship in intact twitching cardiac muscle: direct evidence for modulation by isoproterenol and EMD 53998. Biophys J. 1995 Jul;69(1):189–201. doi: 10.1016/S0006-3495(95)79889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni C., Hano O., Ventura C., Lakatta E. G., Klockow M., Spurgeon H., Capogrossi M. C. A novel positive inotropic substance enhances contractility without increasing the Ca2+ transient in rat myocardium. J Mol Cell Cardiol. 1991 Mar;23(3):325–331. doi: 10.1016/0022-2828(91)90068-w. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension responses to sudden length change in stimulated frog muscle fibres near slack length. J Physiol. 1977 Jul;269(2):441–515. doi: 10.1113/jphysiol.1977.sp011911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambassi G., Capogrossi M. C., Klockow M., Lakatta E. G. Enantiomeric dissection of the effects of the inotropic agent, EMD 53998, in single cardiac myocytes. Am J Physiol. 1993 Mar;264(3 Pt 2):H728–H738. doi: 10.1152/ajpheart.1993.264.3.H728. [DOI] [PubMed] [Google Scholar]
- Gross T., Lues I., Daut J. A new cardiotonic drug reduces the energy cost of active tension in cardiac muscle. J Mol Cell Cardiol. 1993 Mar;25(3):239–244. doi: 10.1006/jmcc.1993.1030. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Stewart A., Sosa H., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994 Dec;67(6):2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft T., Chalovich J. M., Yu L. C., Brenner B. Parallel inhibition of active force and relaxed fiber stiffness by caldesmon fragments at physiological ionic strength and temperature conditions: additional evidence that weak cross-bridge binding to actin is an essential intermediate for force generation. Biophys J. 1995 Jun;68(6):2404–2418. doi: 10.1016/S0006-3495(95)80423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft T., Yu L. C., Kuhn H. J., Brenner B. Effect of Ca2+ on weak cross-bridge interaction with actin in the presence of adenosine 5'-[gamma-thio]triphosphate). Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11362–11366. doi: 10.1073/pnas.89.23.11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. J., Güth K., Drexler B., Berberich W., Rüegg J. C. Investigation of the temperature dependence of the cross bridge parameters for attachment, force generation and detachment as deduced from mechano-chemical studies in glycerinated single fibres from the dorsal longitudinal muscle of Lethocerus maximus. Biophys Struct Mech. 1979 Dec;6(1):1–29. doi: 10.1007/BF00537592. [DOI] [PubMed] [Google Scholar]
- Lee J. A., Allen D. G. EMD 53998 sensitizes the contractile proteins to calcium in intact ferret ventricular muscle. Circ Res. 1991 Oct;69(4):927–936. doi: 10.1161/01.res.69.4.927. [DOI] [PubMed] [Google Scholar]
- Leijendekker W. J., Herzig J. W. Reduction of myocardial cross-bridge turnover rate in presence of EMD 53998, a novel Ca(2+)-sensitizing agent. Pflugers Arch. 1992 Jul;421(4):388–390. doi: 10.1007/BF00374228. [DOI] [PubMed] [Google Scholar]
- Lues I., Beier N., Jonas R., Klockow M., Haeusler G. The two mechanisms of action of racemic cardiotonic EMD 53998, calcium sensitization and phosphodiesterase inhibition, reside in different enantiomers. J Cardiovasc Pharmacol. 1993 Jun;21(6):883–892. doi: 10.1097/00005344-199306000-00006. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. Addition of phosphate to active muscle fibers probes actomyosin states within the powerstroke. Pflugers Arch. 1989 May;414(1):73–81. doi: 10.1007/BF00585629. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Gambassi G., Warshaw D. M., Keller M. R., Spurgeon H. A., Beier N., Lakatta E. G. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993 Dec;73(6):981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- Strauss J. D., Bletz C., Rüegg J. C. The calcium sensitizer EMD 53998 antagonizes phosphate-induced increases in energy cost of isometric tension in cardiac skinned fibres. Eur J Pharmacol. 1994 Feb 3;252(2):219–224. doi: 10.1016/0014-2999(94)90600-9. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Lee J. A., Shah N., Orchard C. H. Differential effects of the optical isomers of EMD 53998 on contraction and cytoplasmic Ca2+ in isolated ferret cardiac muscle. Circ Res. 1993 Jul;73(1):61–70. doi: 10.1161/01.res.73.1.61. [DOI] [PubMed] [Google Scholar]
- Yu L. C., Brenner B. Structures of actomyosin crossbridges in relaxed and rigor muscle fibers. Biophys J. 1989 Mar;55(3):441–453. doi: 10.1016/S0006-3495(89)82838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


