Abstract
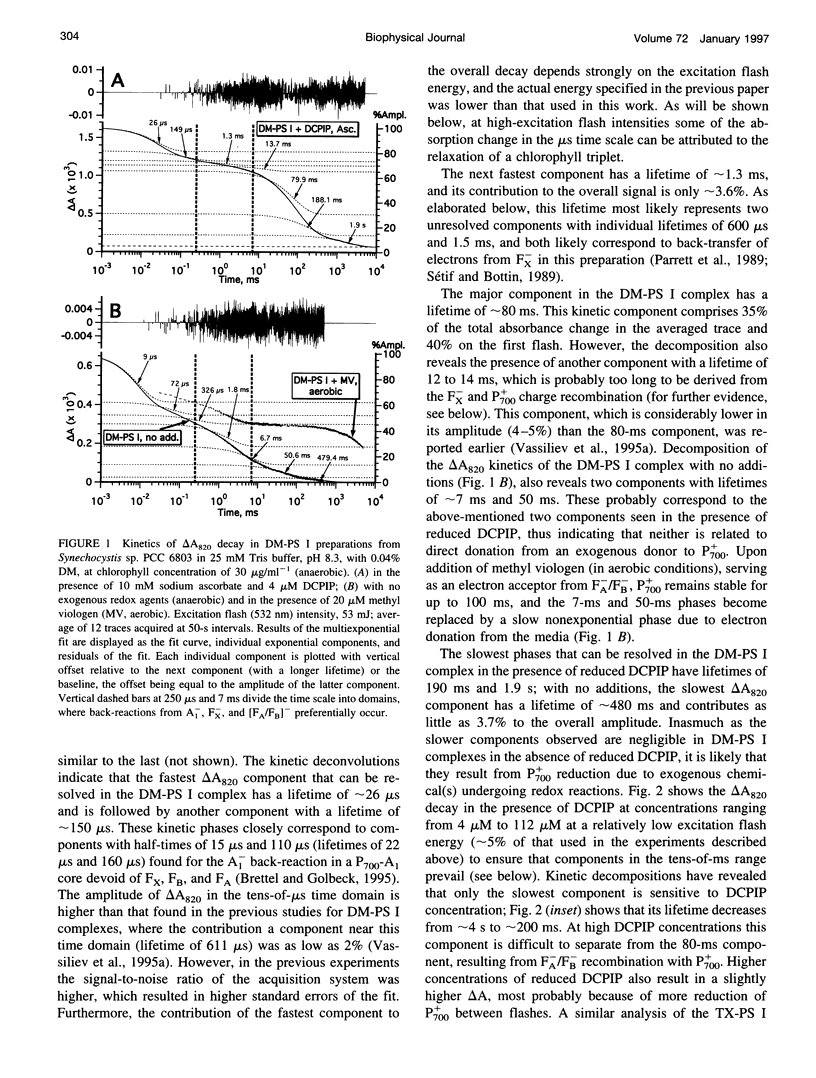
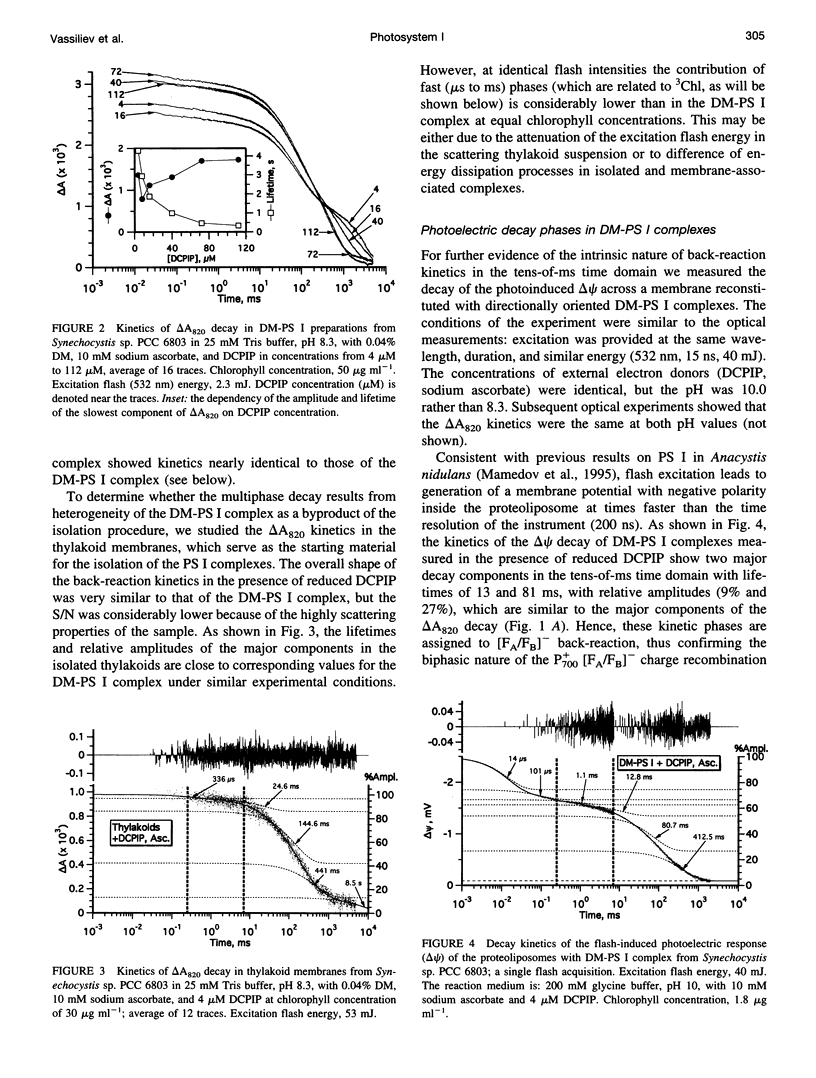
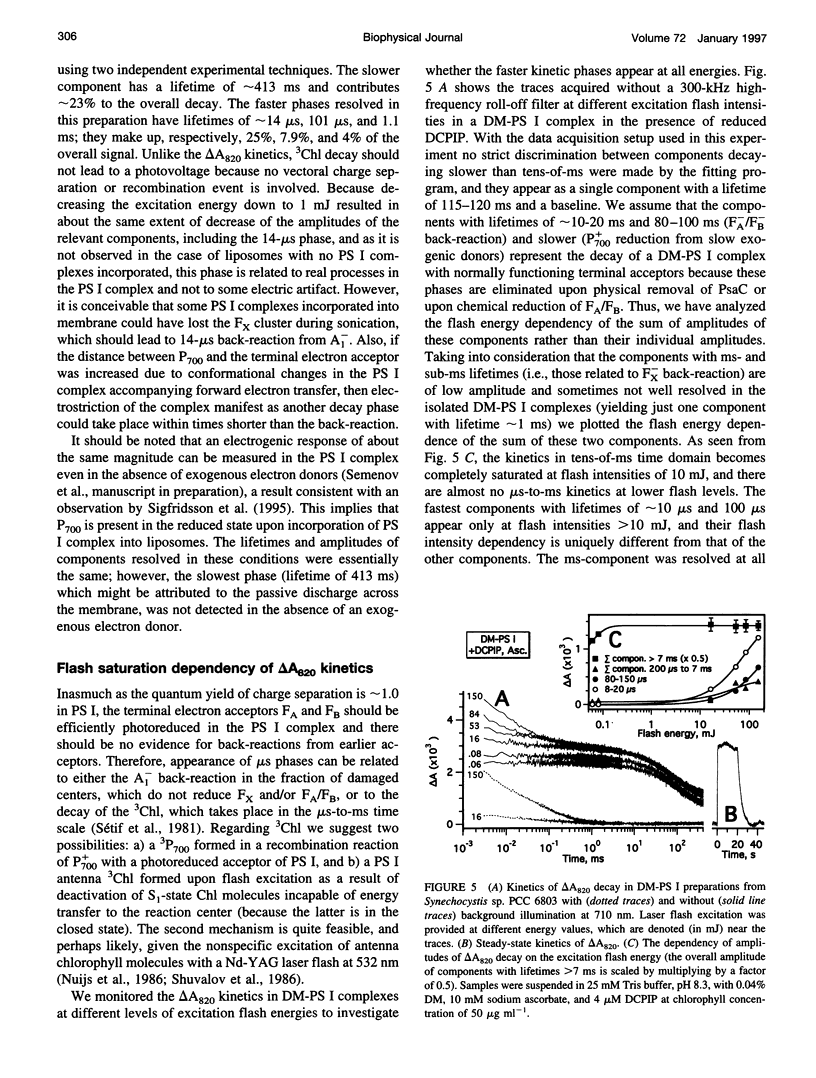
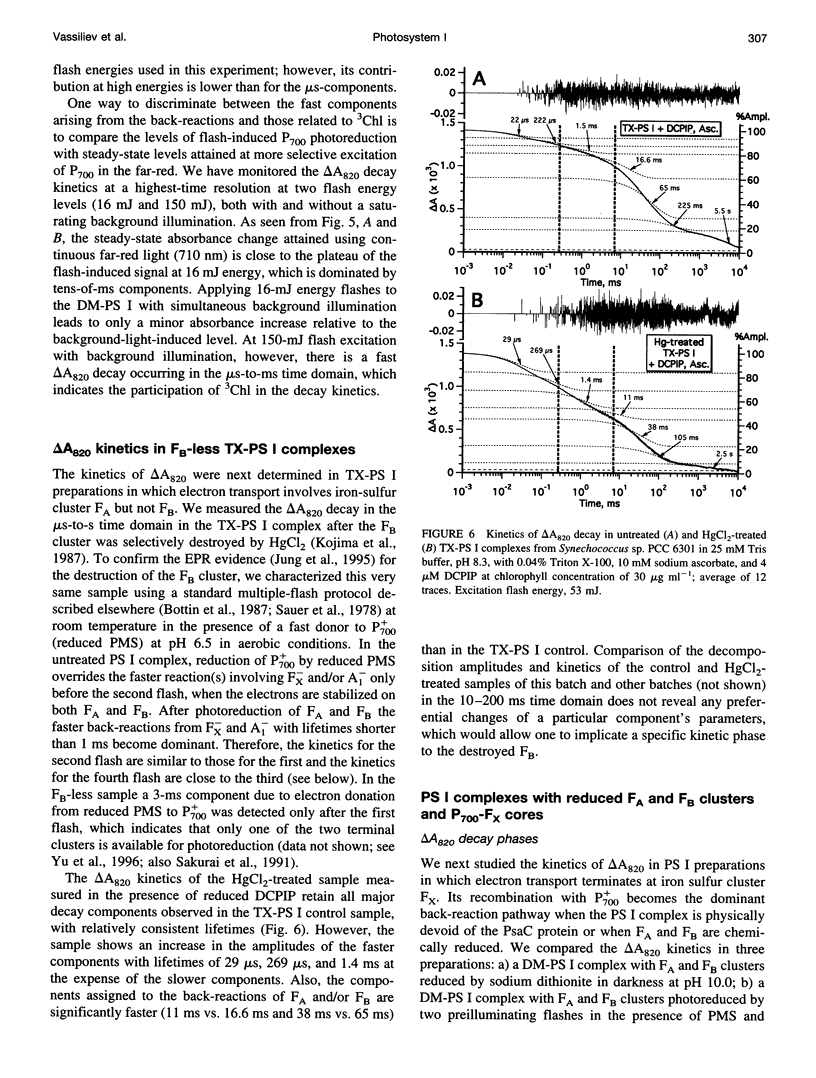
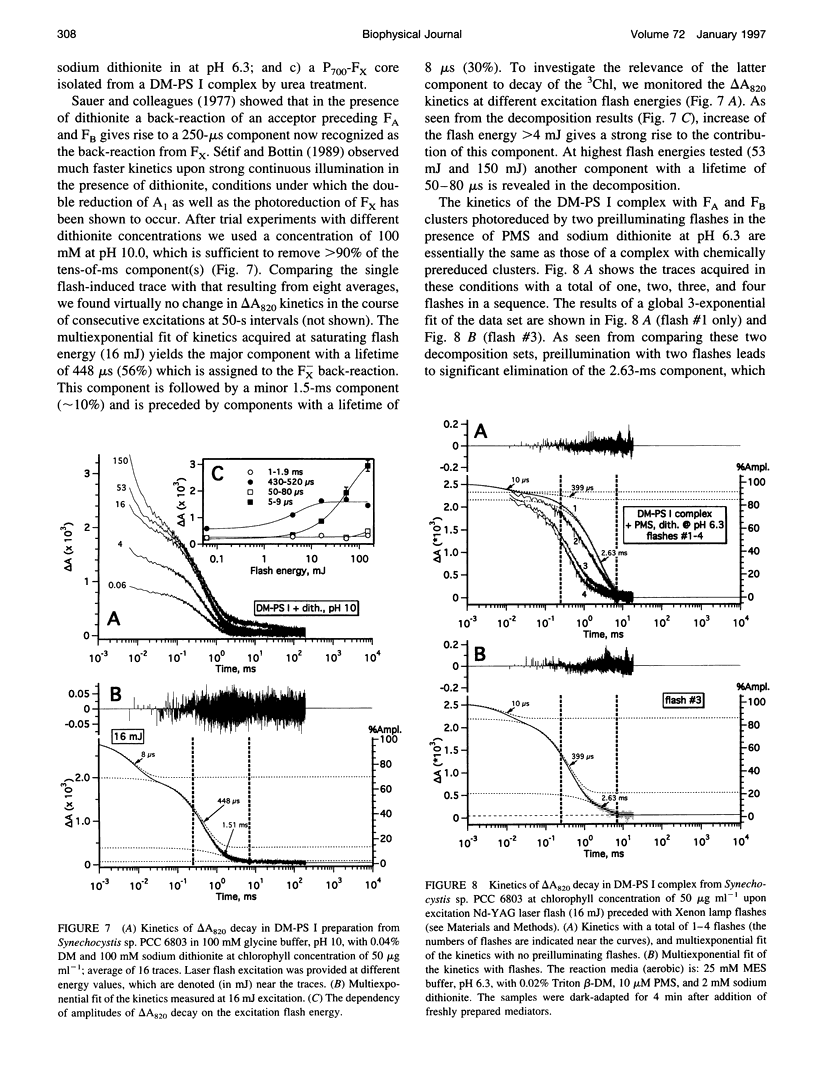
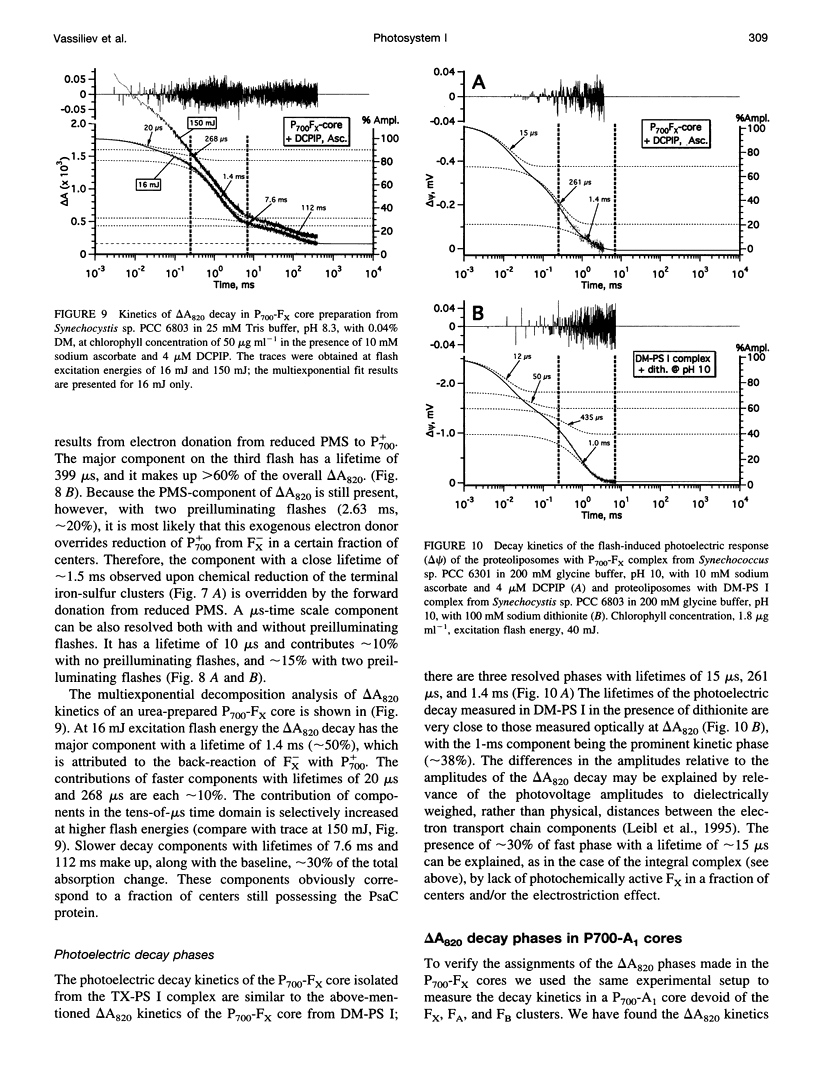
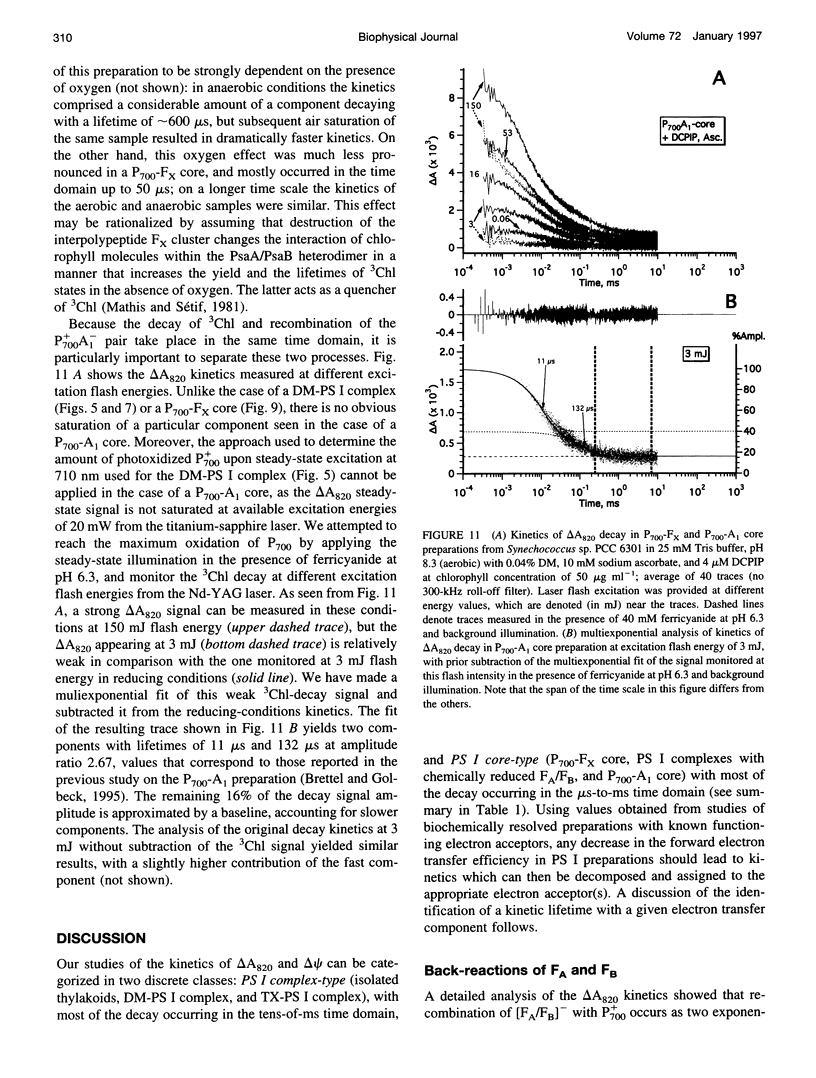
The back-reaction kinetics in Photosystem I (PS I) were studied on the microsecond-to-s time scale in cyanobacterial preparations, which differed in the number of iron-sulfur clusters to assess the contributions of particular components to the reduction of P700+. In membrane fragments and in trimeric P700-FA/FB complexes, the major contribution to the absorbance change at 820 nm (delta A820) was the back-reaction of FA- and/or FB- with lifetimes of approximately 10 and 80 ms (approximately 10% and 40% relative amplitude). The decay of photoinduced electric potential (delta psi) across a membrane with directionally incorporated P700-FA/FB complexes had similar kinetics. HgCl2-treated PS I complexes, which contain FA but no FB, retain both of these kinetic components, indicating that neither can be assigned uniquely to a specific acceptor. These results suggest that FA- reduces P700+ directly and argue for a rapid electron equilibration between FA and FB, which would eliminate their kinetic distinction in a back-reaction. In PsaC-depleted P700-Fx cores, as well as in P700-FA/FB complexes with chemically reduced FA and FB, the major contribution to the delta A820 and the delta psi decay is a biphasic back-reaction of F-X (approximately 400 microseconds and 1.5 ms) with some contribution from A-1 (approximately 10 microseconds and 100 microseconds), the latter of which is variable depending on experimental conditions. The delta A820 decay in a P700-A1 core devoid of all iron-sulfur clusters comprises two phases with lifetimes of 10 microseconds and 130 microseconds (2.7:1 ratio). The biexponential back-reaction kinetics found for each of the electron acceptors may be related to existence of different conformational states of the PS I complex. In all preparations studied, excitation at 532 nm with flash energies exceeding 10 mJ gives rise to formation of antenna 3Chl, which also contributes to delta A820 decay on the tens-of-microsecond time scale. A distinction between delta A820 components related to back-reactions and to 3Chl decay can be made by analysis of flash saturation dependencies and by measurements of kinetics with preoxidized P700.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. L., McIntosh L. Partial conservation of the 5' ndhE-psaC-ndhD 3' gene arrangement of chloroplasts in the cyanobacterium Synechocystis sp. PCC 6803: implications for NDH-D function in cyanobacteria and chloroplasts. Plant Mol Biol. 1991 Apr;16(4):487–499. doi: 10.1007/BF00023416. [DOI] [PubMed] [Google Scholar]
- Drachev L. A., Kaulen A. D., Semenov A. Y., Severina I. I., Skulachev V. P. Lipid-impregnated filters as a tool for studying the electric current-generating proteins. Anal Biochem. 1979 Jul 1;96(1):250–262. doi: 10.1016/0003-2697(79)90580-3. [DOI] [PubMed] [Google Scholar]
- Hastings G., Hoshina S., Webber A. N., Blankenship R. E. Universality of energy and electron transfer processes in photosystem I. Biochemistry. 1995 Nov 28;34(47):15512–15522. doi: 10.1021/bi00047a017. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Ke B. One-way electron discharge subsequent to the photochemical charge separation in photosystem I. Biochim Biophys Acta. 1972 Jun 23;267(3):595–599. doi: 10.1016/0005-2728(72)90192-2. [DOI] [PubMed] [Google Scholar]
- Leibl W., Toupance B., Breton J. Photoelectric characterization of forward electron transfer to iron-sulfur centers in photosystem I. Biochemistry. 1995 Aug 15;34(32):10237–10244. doi: 10.1021/bi00032a018. [DOI] [PubMed] [Google Scholar]
- Li N., Warren P. V., Golbeck J. H., Frank G., Zuber H., Bryant D. A. Polypeptide composition of the Photosystem I complex and the Photosystem I core protein from Synechococcus sp. PCC 6301. Biochim Biophys Acta. 1991 Aug 23;1059(2):215–225. doi: 10.1016/s0005-2728(05)80206-3. [DOI] [PubMed] [Google Scholar]
- Lüneberg J., Fromme P., Jekow P., Schlodder E. Spectroscopic characterization of PS I core complexes from thermophilic Synechococcus sp. Identical reoxidation kinetics of A1- before and after removal of the iron-sulfur-clusters FA and FB. FEBS Lett. 1994 Jan 31;338(2):197–202. doi: 10.1016/0014-5793(94)80364-1. [DOI] [PubMed] [Google Scholar]
- Parrett K. G., Mehari T., Warren P. G., Golbeck J. H. Purification and properties of the intact P-700 and Fx-containing Photosystem I core protein. Biochim Biophys Acta. 1989 Feb 28;973(2):324–332. doi: 10.1016/s0005-2728(89)80439-6. [DOI] [PubMed] [Google Scholar]
- Rodday S. M., Webber A. N., Bingham S. E., Biggins J. Evidence that the FX domain in photosystem I interacts with the subunit PsaC: site-directed changes in PsaB destabilize the subunit interaction in Chlamydomonas reinhardtii. Biochemistry. 1995 May 16;34(19):6328–6334. doi: 10.1021/bi00019a010. [DOI] [PubMed] [Google Scholar]
- Sauer K., Mathis P., Acker S., van Best J. A. Absorption changes of P-700 reversible in milliseconds at low temperature in Triton-solubilized photosystem I particles. Biochim Biophys Acta. 1979 Mar 15;545(3):466–472. doi: 10.1016/0005-2728(79)90155-5. [DOI] [PubMed] [Google Scholar]
- Sauer K., Mathis P., Acker S., van Best J. A. Electron acceptors associated with P-700 in Triton solubilized photosystem I particles from spinach chloroplasts. Biochim Biophys Acta. 1978 Jul 6;503(1):120–134. doi: 10.1016/0005-2728(78)90166-4. [DOI] [PubMed] [Google Scholar]
- Sigfridsson K., Hansson O., Brzezinski P. Electrogenic light reactions in photosystem I: resolution of electron-transfer rates between the iron-sulfur centers. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3458–3462. doi: 10.1073/pnas.92.8.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart L. B., Anderson S. L., McIntosh L. Targeted genetic inactivation of the photosystem I reaction center in the cyanobacterium Synechocystis sp. PCC 6803. EMBO J. 1991 Nov;10(11):3289–3296. doi: 10.1002/j.1460-2075.1991.tb04893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sétif P., Brettel K. Forward electron transfer from phylloquinone A1 to iron-sulfur centers in spinach photosystem I. Biochemistry. 1993 Aug 10;32(31):7846–7854. doi: 10.1021/bi00082a002. [DOI] [PubMed] [Google Scholar]
- Vassiliev I. R., Jung Y. S., Smart L. B., Schulz R., McIntosh L., Golbeck J. H. A mixed-ligand iron-sulfur cluster (C556SPaB or C565SPsaB) in the Fx-binding site leads to a decreased quantum efficiency of electron transfer in photosystem I. Biophys J. 1995 Oct;69(4):1544–1553. doi: 10.1016/S0006-3495(95)80026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren P. V., Golbeck J. H., Warden J. T. Charge recombination between P700+ and A1- occurs directly to the ground state of P700 in a photosystem I core devoid of FX, FB, and FA. Biochemistry. 1993 Jan 26;32(3):849–857. doi: 10.1021/bi00054a016. [DOI] [PubMed] [Google Scholar]
- Warren P. V., Parrett K. G., Warden J. T., Golbeck J. H. Characterization of a photosystem I core containing P700 and intermediate electron acceptor A1. Biochemistry. 1990 Jul 17;29(28):6545–6550. doi: 10.1021/bi00480a001. [DOI] [PubMed] [Google Scholar]


