Abstract
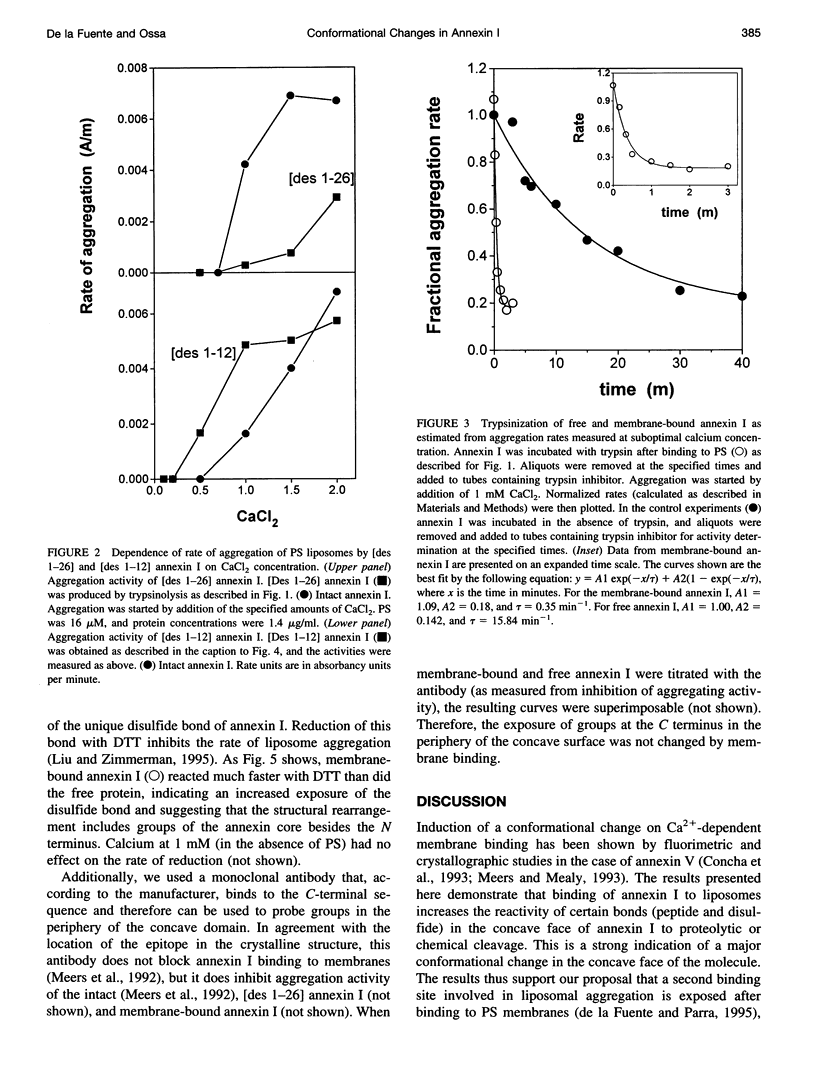
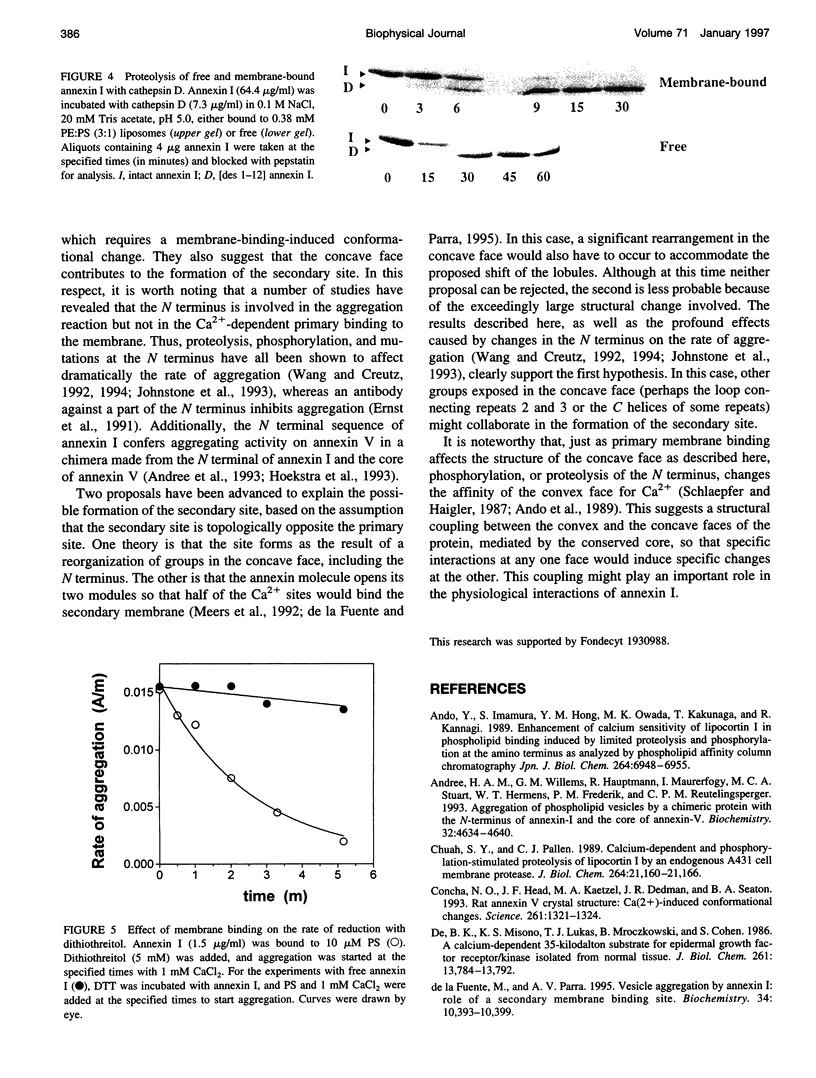
Recent studies have revealed that binding of annexin I to phospholipids induces the formation of a second phospholipid binding site. It is shown that the N terminus on the concave side of membrane-bound annexin I is cleaved much faster by trypsin or cathepsin than the N terminus of the free protein. The reactivity of the unique disulfide bond located near the concave face was similarly increased by membrane binding. These results demonstrate that Ca(2+)-dependent membrane binding induces a conformational change on the concave side of the annexin I molecule and support the notion that this face of the molecule may contribute to the formation of the secondary membrane-binding site.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando Y., Imamura S., Hong Y. M., Owada M. K., Kakunaga T., Kannagi R. Enhancement of calcium sensitivity of lipocortin I in phospholipid binding induced by limited proteolysis and phosphorylation at the amino terminus as analyzed by phospholipid affinity column chromatography. J Biol Chem. 1989 Apr 25;264(12):6948–6955. [PubMed] [Google Scholar]
- Andree H. A., Willems G. M., Hauptmann R., Maurer-Fogy I., Stuart M. C., Hermens W. T., Frederik P. M., Reutelingsperger C. P. Aggregation of phospholipid vesicles by a chimeric protein with the N-terminus of annexin I and the core of annexin V. Biochemistry. 1993 May 4;32(17):4634–4640. doi: 10.1021/bi00068a022. [DOI] [PubMed] [Google Scholar]
- Concha N. O., Head J. F., Kaetzel M. A., Dedman J. R., Seaton B. A. Rat annexin V crystal structure: Ca(2+)-induced conformational changes. Science. 1993 Sep 3;261(5126):1321–1324. doi: 10.1126/science.8362244. [DOI] [PubMed] [Google Scholar]
- Ernst J. D., Hoye E., Blackwood R. A., Mok T. L. Identification of a domain that mediates vesicle aggregation reveals functional diversity of annexin repeats. J Biol Chem. 1991 Apr 15;266(11):6670–6673. [PubMed] [Google Scholar]
- Francis J. W., Balazovich K. J., Smolen J. E., Margolis D. I., Boxer L. A. Human neutrophil annexin I promotes granule aggregation and modulates Ca(2+)-dependent membrane fusion. J Clin Invest. 1992 Aug;90(2):537–544. doi: 10.1172/JCI115892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Johnstone S. A., Hubaishy I., Waisman D. M. Regulation of annexin I-dependent aggregation of phospholipid vesicles by protein kinase C. Biochem J. 1993 Sep 15;294(Pt 3):801–807. doi: 10.1042/bj2940801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee G., de la Fuente M., Pollard H. B. A barium-dependent chromaffin granule aggregating protein from bovine adrenal medulla and other tissues. Ann N Y Acad Sci. 1991;635:477–479. doi: 10.1111/j.1749-6632.1991.tb36532.x. [DOI] [PubMed] [Google Scholar]
- Liu L., Zimmerman U. J. An intramolecular disulfide bond is essential for annexin I-mediated liposome aggregation. Biochem Mol Biol Int. 1995 Feb;35(2):345–350. [PubMed] [Google Scholar]
- Meers P., Mealy T., Pavlotsky N., Tauber A. I. Annexin I-mediated vesicular aggregation: mechanism and role in human neutrophils. Biochemistry. 1992 Jul 21;31(28):6372–6382. doi: 10.1021/bi00143a003. [DOI] [PubMed] [Google Scholar]
- Meers P., Mealy T. Relationship between annexin V tryptophan exposure, calcium, and phospholipid binding. Biochemistry. 1993 May 25;32(20):5411–5418. doi: 10.1021/bi00071a016. [DOI] [PubMed] [Google Scholar]
- Meers P., Mealy T., Tauber A. I. Annexin I interactions with human neutrophil specific granules: fusogenicity and coaggregation with plasma membrane vesicles. Biochim Biophys Acta. 1993 Apr 22;1147(2):177–184. doi: 10.1016/0005-2736(93)90002-h. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Tokuda M., Masaki T., Fujimura T., Tai Y., Itano T., Matsui H., Ishida T., Konishi R., Takahara J. Involvement of annexin-I in glucose-induced insulin secretion in rat pancreatic islets. Endocrinology. 1995 Jun;136(6):2421–2426. doi: 10.1210/endo.136.6.7750463. [DOI] [PubMed] [Google Scholar]
- Peers S. H., Flower R. J. The role of lipocortin in corticosteroid actions. Am Rev Respir Dis. 1990 Feb;141(2 Pt 2):S18–S21. [PubMed] [Google Scholar]
- Raynal P., Pollard H. B. Annexins: the problem of assessing the biological role for a gene family of multifunctional calcium- and phospholipid-binding proteins. Biochim Biophys Acta. 1994 Apr 5;1197(1):63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Characterization of Ca2+-dependent phospholipid binding and phosphorylation of lipocortin I. J Biol Chem. 1987 May 15;262(14):6931–6937. [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait J. F., Sakata M., McMullen B. A., Miao C. H., Funakoshi T., Hendrickson L. E., Fujikawa K. Placental anticoagulant proteins: isolation and comparative characterization four members of the lipocortin family. Biochemistry. 1988 Aug 23;27(17):6268–6276. doi: 10.1021/bi00417a011. [DOI] [PubMed] [Google Scholar]
- Wang W., Creutz C. E. Regulation of the chromaffin granule aggregating activity of annexin I by phosphorylation. Biochemistry. 1992 Oct 20;31(41):9934–9939. doi: 10.1021/bi00156a011. [DOI] [PubMed] [Google Scholar]
- Wang W., Creutz C. E. Role of the amino-terminal domain in regulating interactions of annexin I with membranes: effects of amino-terminal truncation and mutagenesis of the phosphorylation sites. Biochemistry. 1994 Jan 11;33(1):275–282. doi: 10.1021/bi00167a036. [DOI] [PubMed] [Google Scholar]
- Weng X., Luecke H., Song I. S., Kang D. S., Kim S. H., Huber R. Crystal structure of human annexin I at 2.5 A resolution. Protein Sci. 1993 Mar;2(3):448–458. doi: 10.1002/pro.5560020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaks W. J., Creutz C. E. Evaluation of the annexins as potential mediators of membrane fusion in exocytosis. J Bioenerg Biomembr. 1990 Apr;22(2):97–120. doi: 10.1007/BF00762942. [DOI] [PubMed] [Google Scholar]




